
The “COVID-19 Antigen Rapid Test – Anterior Nasal Swab” and the “COVID-19 Antigen Rapid Test (Colloidal Gold) – Saliva” developed independently by JOINSTAR Biomedical Technology Co., Ltd obtained the EU CE certification for self-testing on January 20th and February 2nd, issued by the CE 1434.
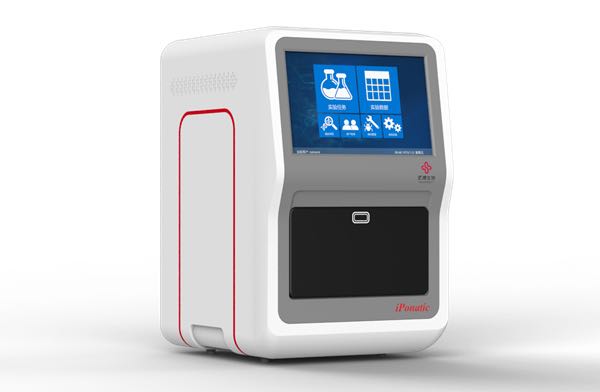
On January 4th, the four-channel iPonatic nucleic acid detection analyzer of Sansure Biotech was certified by FDA. This is another global authority certification obtained after the approval of China NMPA and the European Union CE certification.

Siemens Healthineers said Tuesday that the TüV Rheinland certification agency has certified its first products under the new European In Vitro Diagnostic Regulation.
✔ All (13)
✔ Press release (0)
✔ Industry news (13)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
