

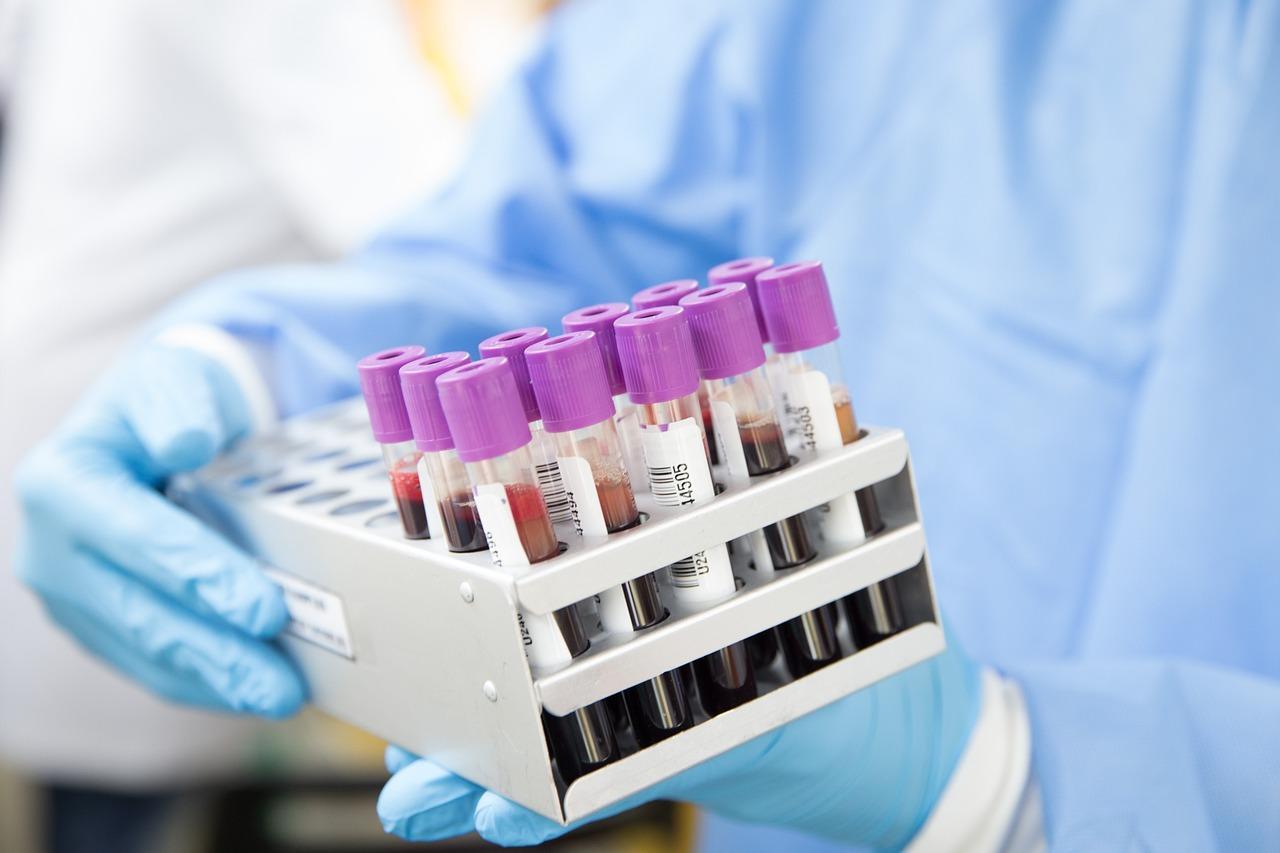
Blood typing reagents mainly refer to reagents and kits used separately or in combination with blood grouping analyzer, which belong to a segment of in vitro diagnostic (IVD) reagent industry.
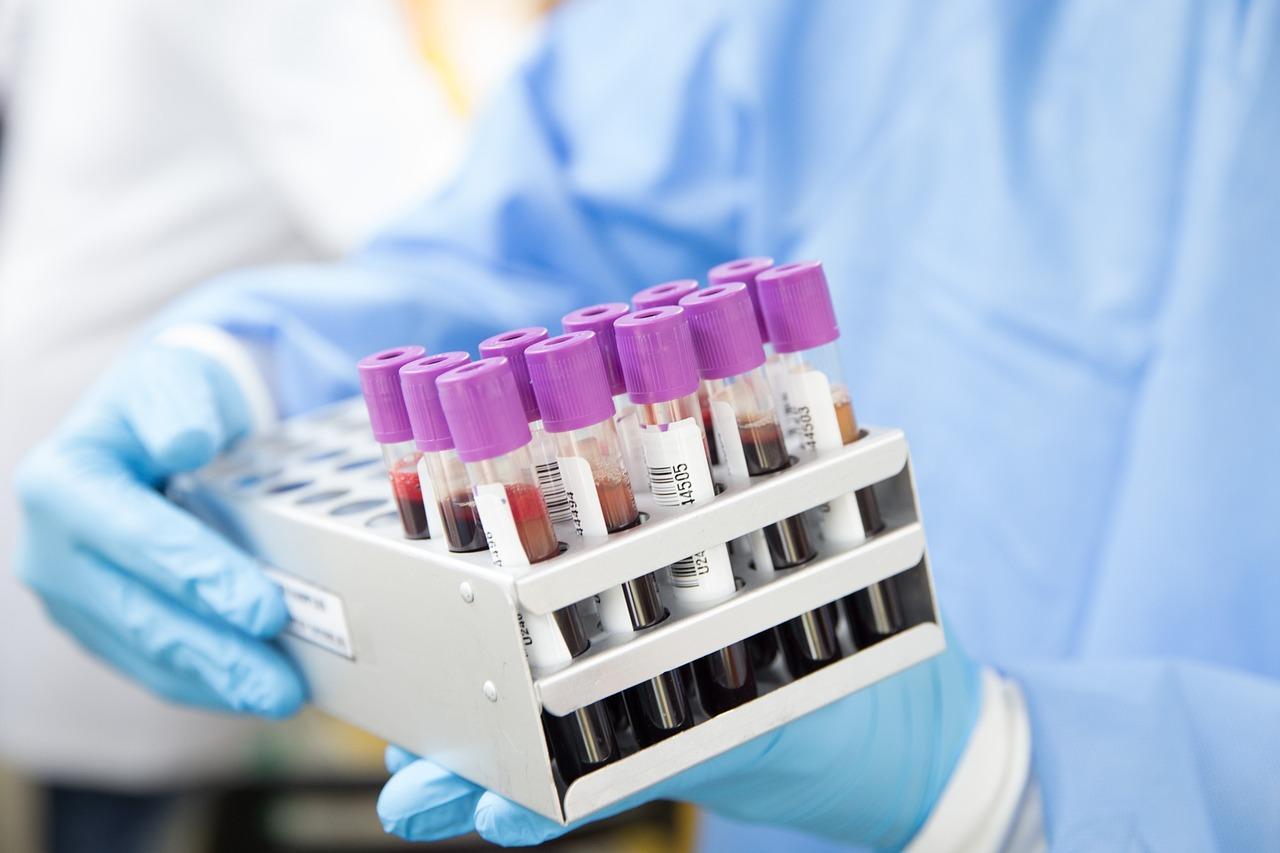
Automatic blood grouping analyzer is an analyzer that can forward and reverse ABO blood group, Rh (D) blood group test, irregular antibody screening and cross-matching of blood test. It mainly consists of liquid handling system, incubator, centrifuge, interpretoscope, and microcomputer system.

PathAI, Inc., a global leader in artificial intelligence (AI)-powered pathology solutions, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to PathAssist Derm1, designed to analyze digital pathology whole slide images (WSIs) of skin lesions and aid pathologists in their review.

Quanterix Corporation (NASDAQ: QTRX), a company fueling scientific discovery through ultra-sensitive biomarker detection, today announced financial results for the fourth quarter and full year ended December 31, 2025.

SOPHiA GENETICS (Nasdaq: SOPH), a global leader in AI-driven precision medicine, today provided preliminary unaudited financial results for the fourth quarter and full year 2025, initiated its financial outlook for 2026, and announced an executive transition plan, including the promotion of Ross Muken to Chief Executive Officer (CEO), effective July 1, 2026, and the transition of co-Founder Jurgi Camblong to Executive Chairman.

Natera, Inc. (NASDAQ: NTRA), a global leader in cell-free DNA and genetic testing, today reported its financial results for the fourth quarter and full year ended December 31, 2025.

Agilent Technologies, Inc. (NYSE: A) today reported revenue of $1.80 billion for the first quarter ended Jan. 31, 2026, representing growth of 7.0% reported and up 4.4% core(1) compared with the first quarter of 2025.

Diasorin (FTSE MIB: DIA) today announces the U.S. FDA approval of the LIAISON QuantiFERON-TB Gold Plus II assay as a next-generation automated Interferon Gamma Release Assay (IGRA) designed in partnership with QIAGEN N.V. (NYSE: QGEN; Frankfurt Prime Standard: QIA) to enhance laboratory productivity, workflow efficiency and turnaround time for latent tuberculosis infection (LTBI) testing in the United States.

Meridian Bioscience, Inc., a leading global provider of diagnostic testing solutions and life science raw materials, today announced that a broad portfolio of its Alethia molecular assays has successfully achieved CE marking under the European In Vitro Diagnostic Regulation (IVDR) (EU) 2017/746.

Guardant Health has acquired MetaSight Diagnostics for $59 million in upfront cash to bolster its multi-disease detection pipeline, the company said Thursday. The deal includes up to $90 million in payments tied to future commercial performance and regulatory approvals.

Merck (MRK.N), opens new tab said on Monday it would create a separate division for its cancer business, anchored by its top-selling drug Keytruda, as the U.S. drugmaker braces for the upcoming patent expiry of the blockbuster treatment.

Quest Diagnostics Incorporated (NYSE: DGX), a leading provider of diagnostic information services, announced today financial results for the fourth quarter and full year ended December 31, 2025.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
