


Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

BD (Becton, Dickinson and Company) (NYSE: BDX) ("BD" or the "Company") today announced that the Company's Board of Directors has set the close of business on February 5, 2026, as the record date for the previously announced spin-off of BD's Biosciences & Diagnostic Solutions business to BD's shareholders. Immediately following the spin-off, the spun-off entity will be combined with Waters Corporation (NYSE: WAT) ("Waters") in a Reverse Morris Trust transaction. The combination is expected to be completed on February 9, 2026, subject to the satisfaction of customary closing conditions.

The instruments employed by hospitals participating in the basic study were mainly from various manufacturers such as Sysmex, Stago, Werfen, Sekisui, Beckman, Mindray, Succeeder, BSBE, and Rayto, involving 96 instruments, of which 11 hospitals used instruments from two manufacturers, with the use of each brand of blood coagulation analyzers shown in Fig. 4.

The Fujian Agriculture and Forestry University in East China has unveiled the country's first humanoid diagnostic and treatment robot that deeply integrates non-invasive brain computer interface (BCI) technology. The robot is expected to help address long-standing challenges in early intervention of autism and other neurodevelopmental disorders, bringing good hope to the rehabilitation of more than 13 million autism patients in China, Global Times learned from the robot developers on Sunday.

Diasorin (FTSE MIB: DIA) announced today that, following the recent 510(k) clearance and CLIA-waiver from the U.S. Food and Drug Administration (FDA) for its first assay—the FLU A/B, RSV & COVID-19 Panel for use on the LIAISON NES® platform— the Company has entered into an exclusive distribution agreement with Fisher Scientific, part of Thermo Fisher Scientific, for the LIAISON NES® molecular point-of-care (POC) platform in the U.S. hospital market.
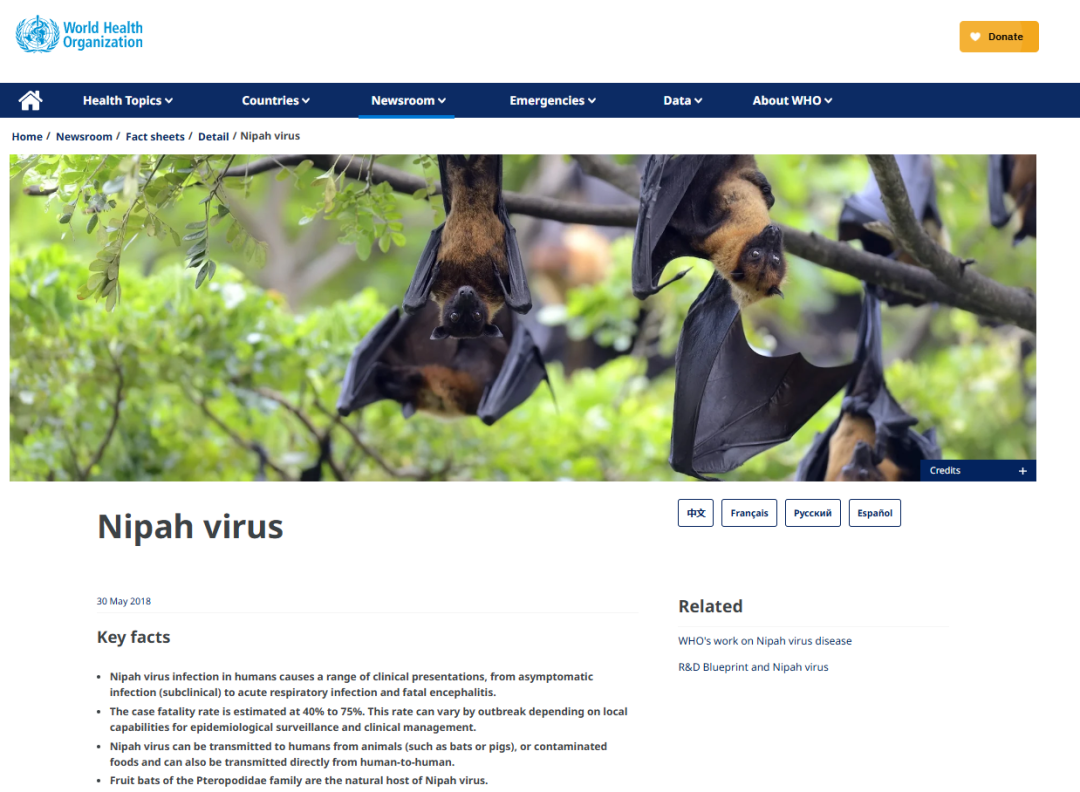
Recently, an outbreak of Nipah virus occurred in West Bengal, India, with 5 confirmed cases reported, including several healthcare workers. Nearly 100 people have been placed under home quarantine, and one patient is in critical condition, highlighting the urgency of epidemic control.

Qure.ai, a global digital health company focused on AI-led medical diagnostics, has received a multi-million-dollar grant from the Gates Foundation to support the development of AI-enabled point-of-care ultrasound and create an open-source multi-modal health database aimed at improving lung disease detection in underserved regions.

Merck (MRK.N), opens new tab is no longer in discussions to buy cancer drug developer Revolution Medicines (RVMD.O), opens new tab, the Wall Street Journal reported on Sunday.

Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced that the U.S. Food and Drug Administration (FDA) has approved Guardant360® CDx as a companion diagnostic to identify patients with BRAF V600E-mutant metastatic colorectal cancer (mCRC) who may benefit from treatment with BRAFTOVI® (encorafenib) in combination with cetuximab and chemotherapy in accordance with the approved product labeling.

HORIBA, a global leader in analytical and measurement technology, has obtained CE IVDR certification for its new Yumizen H500 CRP benchtop hematology analyzer which delivers a simultaneous 5-part extended differential (DIFF) and rapid C-Reactive Protein (CRP) whole blood measurement. Based on its award winning Yumizen H500 compact analyzer, the new instrument is designed for small laboratories and ideal for use in a variety of hospital testing settings for rapid and highly accurate detection of infection and inflammation.

BIOMAKERS, a precision medicine and oncology intelligence company headquartered in San Francisco, today announced the closing of an additional $8 million. The financing marks a key inflection point as the company transitions from building a differentiated global diagnostics and data infrastructure to scaling an AI-native platform – while continuing to expand its comprehensive molecular testing reach across Latin America – designed to accelerate drug and diagnostic development, improve clinical trial execution, and enable precision oncology at global scale.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
