
The US Food and Drug Administration said on Tuesday it has granted Emergency Use Authorization for DiaCarta's monkeypox PCR test.
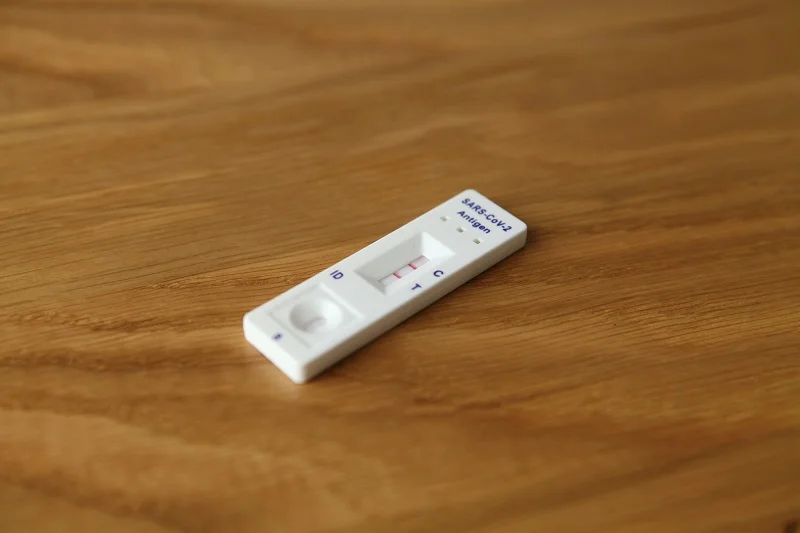
Jiangsu Huadong Medical Device Industrial and Getein Biotech have signed an agreement to jointly manufacture and sell antigen test kits for Covid-19 and other in vitro diagnostics (IVD) products.

Becton, Dickinson and Company (BD) and CerTest Biotec have announced that their molecular assay for detecting the Mpox virus has received Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA).

Genomic and diagnostic testing company Prenetics Global said Tuesday that the US Food and Drug Administration has cleared ACTOnco, a comprehensive genomic profiling test for solid tumors.

Precision BioLogic said Thursday it secured US Food and Drug Administration 510(k) premarket clearance for its Cryocheck Chromogenic Factor IX test for managing hemophilia B.

Foundation Medicine said Wednesday its FoundationOne Liquid CDx test has received approval from the US Food and Drug Administration as a companion diagnostic for Genentech's Rozlytrek (entrectinib).

Liquid biopsy oncology firm Burning Rock said on Tuesday that the U.S. Food and Drug Administration (FDA) has granted breakthrough device designation for its OverC Multi-Cancer Detection Blood Test (MCDBT).

Roche Diagnostics China and Beijing Hotgene Biotechnology Co., Ltd. have reached a cooperation to jointly launch the novel coronavirus (2019-nCoV) antigenic detection kit on the basis of fully integrating the advantages of technology and resources of both sides.

Visby Medical's respiratory panel test has received Emergency Use Authorization from the US Food and Drug Administration, according to a letter from the agency.

The US Food and Drug Administration has granted Emergency Use Authorization for Becton Dickinson’s monkeypox PCR test.
✔ All (509)
✔ Press release (3)
✔ Industry news (506)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
