
Roche and Pfizer have launched a collaboration in the U.S. to help those who test positive for COVID-19 find the best resources for the best possible outcomes.

The independently developed "COVID-19 antigen detection kit SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit (Colloidal gold method)" by Synthgene Medical Technology was approved by the UK 5-year CTDA certification.

On December 13, COVID-19 specific medicine was available online. The COVID-19 Consultation Clinic at 111, Inc. Internet hospital has begun pre-selling Pfizer's COVID-19 oral antiviral drug Paxlovid (nematovir/ritonavir tablets), priced at CNY 2,980 per box.

The government of Hong Kong has just announced that effective Wednesday, December 14th 2022, almost all restrictions for international travelers will be removed, which includes restrictions on movement and the issuance of the Amber (probation-like) health code via app.
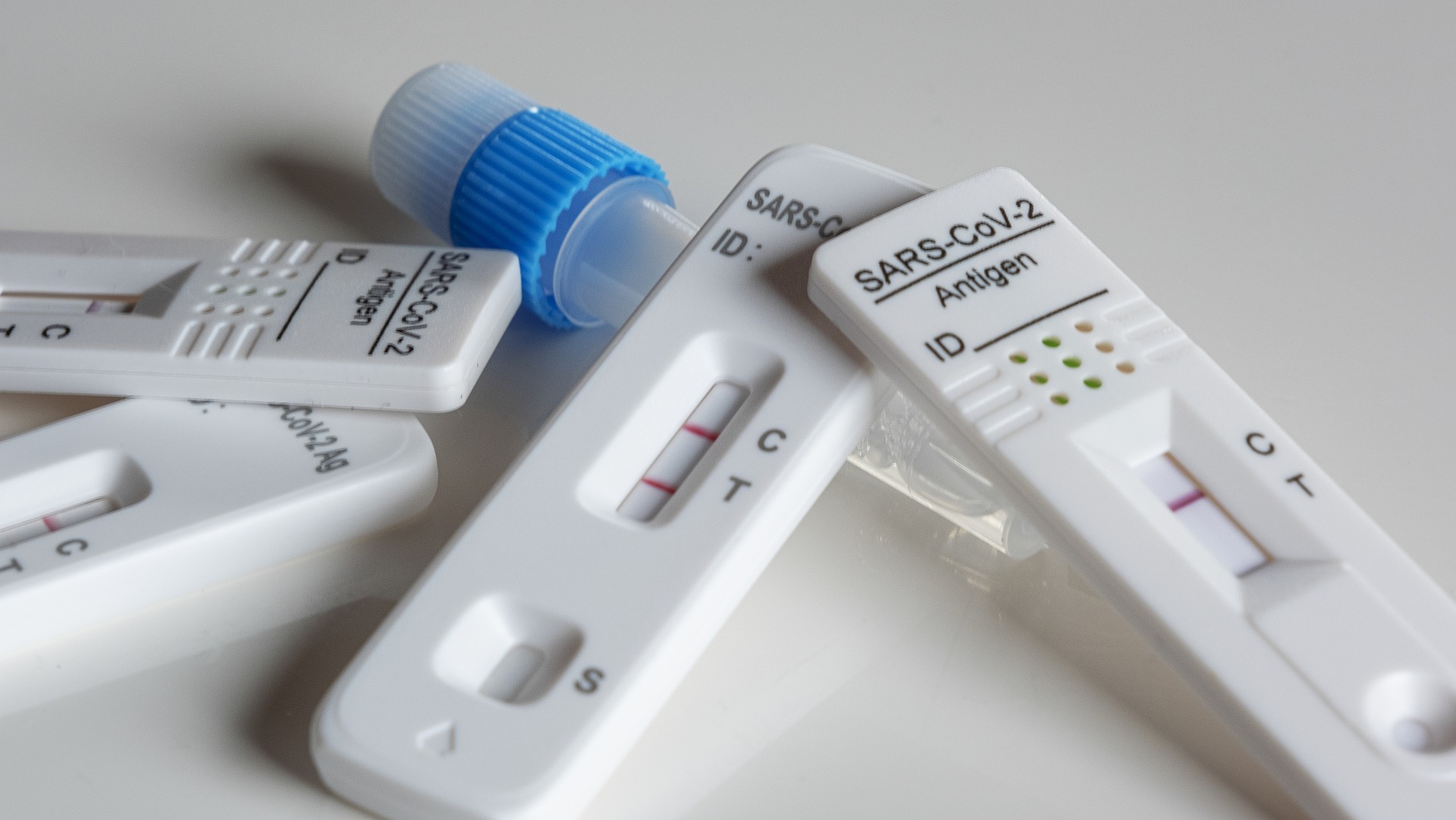
The data shows that the current average daily sales volume of self-test boxes has increased by more than 400 times compared with November.
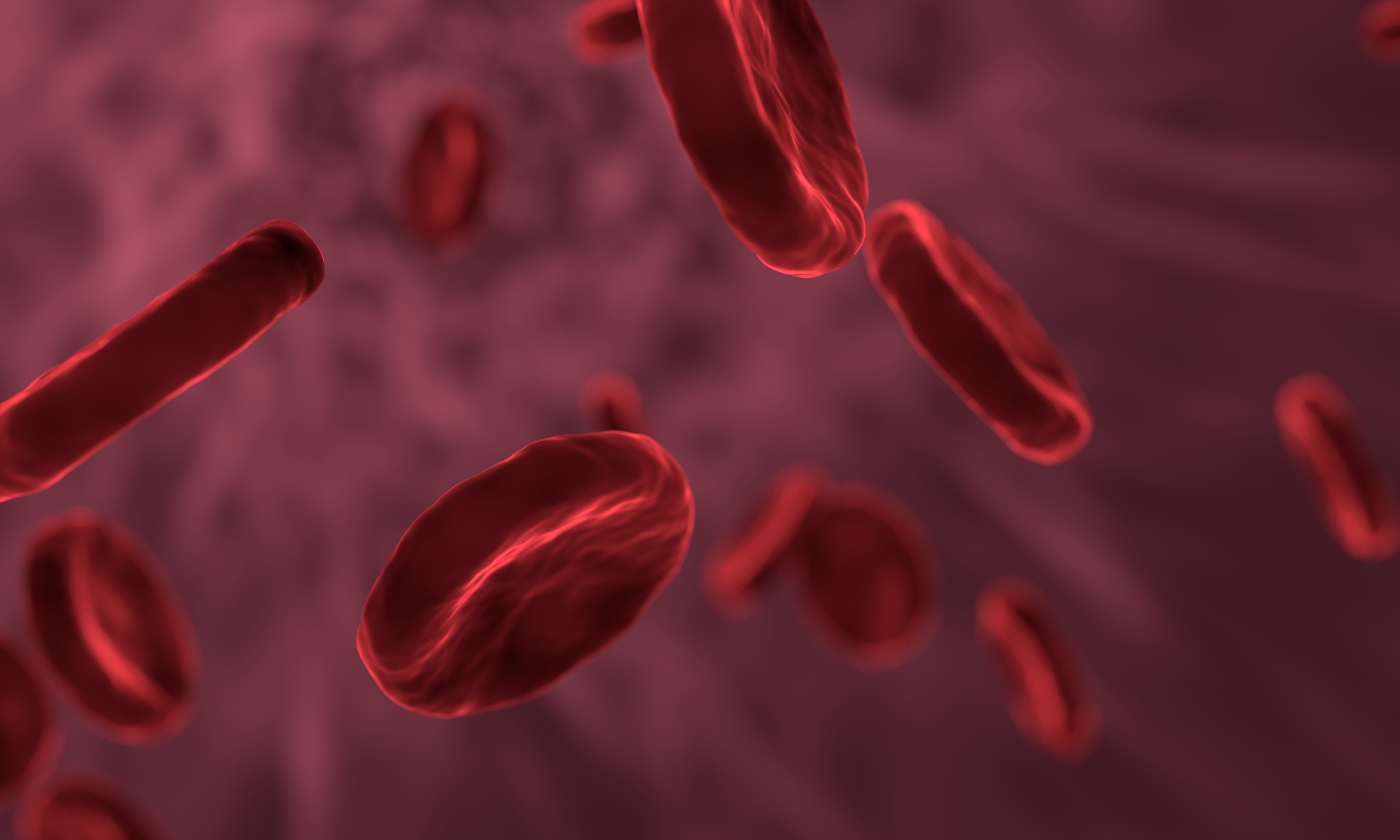
A team has identified cell type-specific gene expression signatures in blood samples from individuals with acute SARS-CoV-2 infections that appeared to predict the risk and types of long-term symptoms they may experience

Roche announced on Thursday that two of its tests for Alzheimer's disease have received 510(k) clearance from the US Food and Drug Administration.

The US Food and Drug Administration this week granted Emergency Use Authorization to Diazyme Laboratories for a SARS-CoV-2 total antibody test.
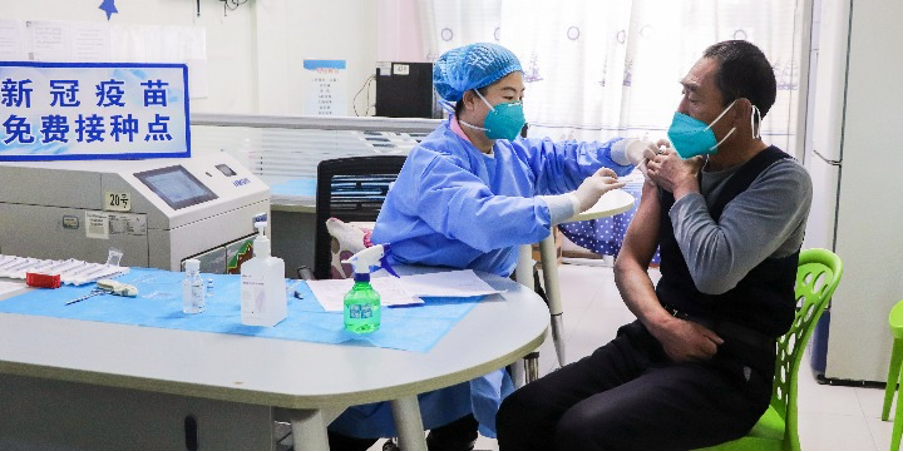
Editor's note: Major adjustments have been made to China's COVID-19 control policies, according to a 10-point notice released by the National Health Commission on Wednesday. Below are some of the key changes, to help you keep up to date.

Thermo Fisher Scientific said on Wednesday that its SeCore CDx HLA Sequencing System was granted de novo classification by the US Food and Drug Administration as a companion diagnostic to Immunocore’s Kimmtrak (tebentafusp-tebn) therapy for uveal melanoma.
✔ All (509)
✔ Press release (3)
✔ Industry news (506)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
