
On April 13, the SARS-CoV-2 Rapid Antigen Test (immunochromatography Assay) developed by Sansure Biotech Inc. has obtained CE certification! This means that the test can be sold in EU countries and countries that recognize EU CE certification.

On April 13, National Medical Products Administrations (NMPA) reviewed and approved Zhuhai Livzon Diagnostics Inc. and Shanghai BioGerm Medical Technology Co., Ltd's COVID-19 Antigen Test.

The US Food and Drug Administration last week granted separate Emergency Use Authorizations for a PCR-based SARS-CoV-2 test developed by the University of California, San Diego and an at-home nasal specimen collection kit from the Washington D.C. Department of Health.

CLS.CN reported on April 12 that Andon Health announced that the net profit in the first quarter is expected to be CNY 14 billion to CNY 16 billion, a year-on-year increase of 36707.43% to 41965.63%.

The US Food and Drug Administration this week granted separate Emergency Use Authorizations for over-the-counter SARS-CoV-2 antigen self-tests developed by Osang Healthcare.

The scientists, led by Joseph Vinetz, MD, an infectious diseases specialist, were interested to find out if an oral medication used to treat pancreatitis could reduce the viral load (the amount of virus in your body) of SARS-CoV-2 and improve symptoms in people newly diagnosed with COVID-19.

The Biden-Harris Administration announced on April 4 that more than 59 million Americans with Medicare Part B, including those enrolled in a Medicare Advantage plan, now have access to Food and Drug Administration (FDA) approved, authorized, or cleared OTC COVID-19 tests at no cost.
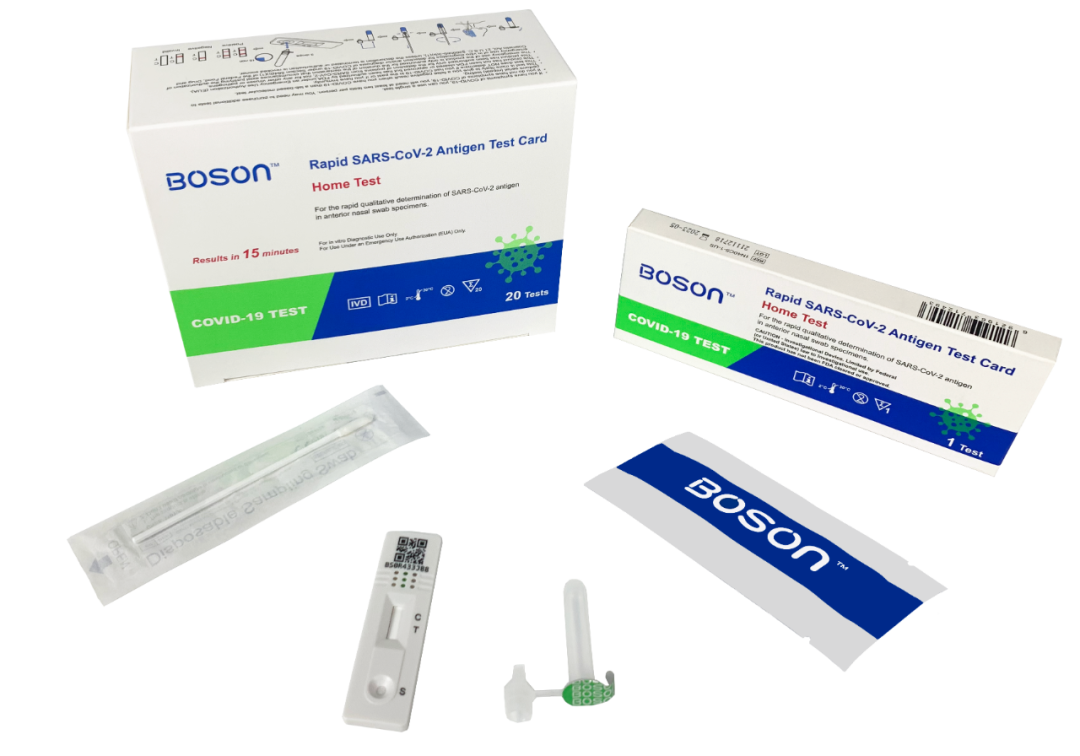
On April 6, it was reported that the Rapid SARS-CoV-2 Antigen Test Card produced by Xiamen Boson Biotech Co., Ltd. has obtained the FDA Emergency Use Authorization (EUA).

Jinan Babio Biotechnology Co., Ltd. recently received an official notification from the EU Notified Body for its self-tested novel coronavirus antigen detection product, and subsequently obtained the CE certification issued by PCBC.

Israeli diagnostics firm Todos Medical on Friday reported a nearly 13 percent year-over-year increase in revenues for the fourth quarter on increased demand for its COVID-19 testing products and services, as well as nutritional supplements.
✔ All (509)
✔ Press release (3)
✔ Industry news (506)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
