
Abbott (NYSE: ABT) today announced a unique global partnership with Medtronic to collaborate on an integrated continuous glucose monitoring (CGM) system based on Abbott's most advanced, world-leading FreeStyle Libre technology that will connect with Medtronic's automated insulin delivery (AID) and smart insulin pen systems.

On June 13, 2024, Mindray and Inpeco have officially signed a strategic cooperation agreement at Mindray's headquarters in Shenzhen, China, marking a new era of innovation and collaboration in the in vitro diagnostics industry. This collaboration is founded on the companies’ mutual excellence in their respective fields, as well as their shared values and vision for the future.

Agilent Technologies Inc. (NYSE: A) today announced the launch of the Advanced Dilution System, the ADS 2, a new automation workflow solution that will increase productivity, lower cost of ownership, and improve the overall efficiency within the laboratory.
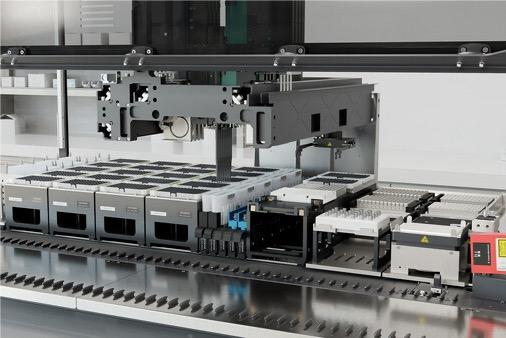
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, announced a collaboration agreement with Hamilton, a leading global manufacturer of laboratory automation technology, to develop automated applications together with robotics-compatible reagent kits to enable greater standardization and reduced human error when conducting large-scale single-cell multiomics experiments.

Agilent Technologies Inc. (NYSE: A) today announced the release of a new automated parallel capillary electrophoresis system for protein analysis – the Agilent ProteoAnalyzer system – at the 23rd Annual PepTalk Conference, being held January 16-19 in San Diego. This new platform simplifies and improves the efficiency of analyzing complex protein mixtures, a process central to analytical workflows across the pharma, biotech, food analysis, and academia sectors.

Abbott’s new laboratory automation system has received U.S. Food and Drug Administration (FDA) approval, the company announced in a statement.

Thermo Fisher Scientific Inc., the world leader in serving science, today announced the launch of the Thermo Scientific™ KingFisher™ Apex™ Dx, an automated nucleic acid purification instrument, and Applied Biosystems™ MagMAX™ Dx Viral/Pathogen NA Isolation Kit for the isolation and purification of viral and bacterial pathogens from respiratory biological specimens. Together these products provide laboratories with an in vitro diagnostic (IVD) and in vitro diagnostic regulation (IVD-R) approved automated sample preparation solutions for increased confidence in downstream results.

Various automated immunoassay analyzers were applied in clinical lab using different principles, which offered great convenience to our daily work in the laboratory, with more stability and accuracy than manual operation.

Becton, Dickinson and Company (BD) has globally introduced the new automated instrument, the BD FACSDuet Premium Sample Preparation System, for cellular diagnostic applications.

Liquid handling instrumentation firm Integra Biosciences said that it has acquired Miroculus, a sample prep automation company that has been developing microfluidics-based platforms for next-generation sequencing.
✔ All (22)
✔ Press release (0)
✔ Industry news (22)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
