Orient Gene Expands Global Production Base

Zhejiang Orient Gene Biotech Co., Ltd. has made another significant step in its global localized production base layout. On August 28 (U.S. time), Healgen Scientific LLC, a subsidiary of Orient Gene, held a ribbon-cutting ceremony for the opening of its production facility in Houston, USA. The ceremony was attended by U.S. Congressman Al Green, Texas State Representative Gene Wu, and Houston City Treasurer Chris Hollins. Once operational, the Houston facility will locally produce respiratory-related products, as well as drug abuse detection reagents. In the future, based on market demand and orders from the U.S. and other American markets, the company plans to further expand localized production capacity, with a designed daily output of millions of units.
Bioperfectus' Two Products Selected for the "Global Fund Mpox Molecular Diagnostic Quality Assurance Standard" List
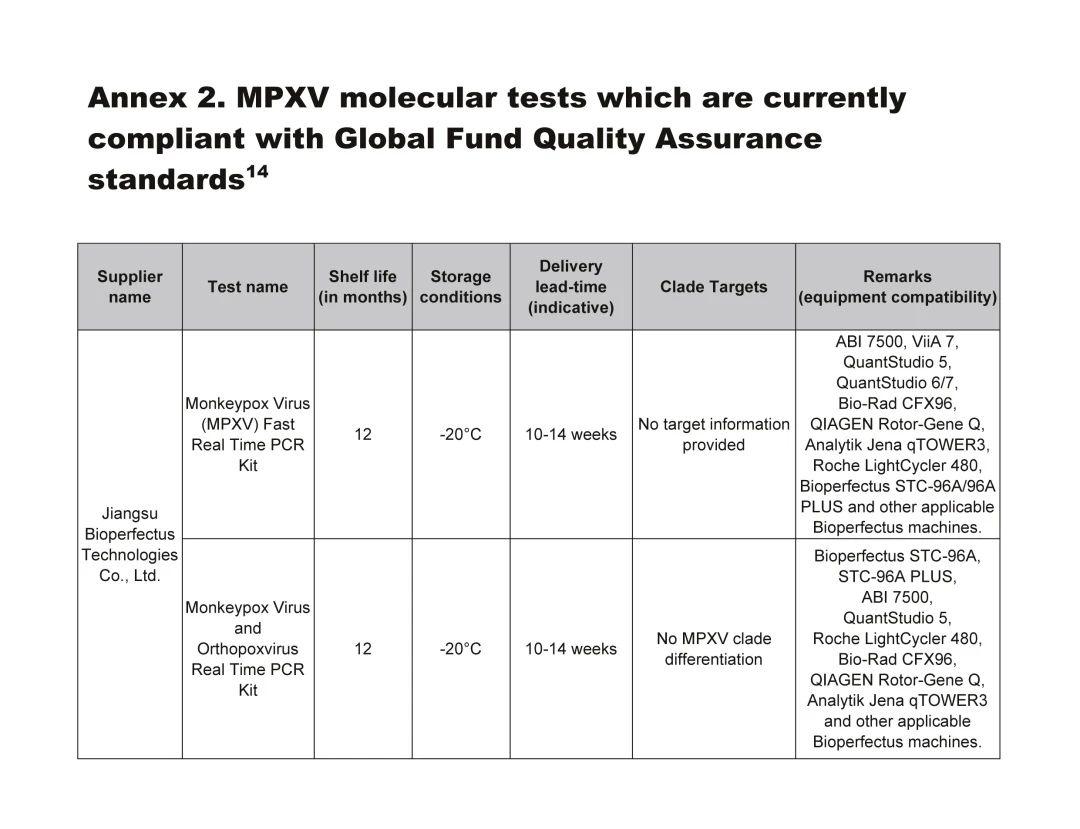
The Global Fund has released the Technical Brief: Prevent, Detect and Respond to Mpox, which includes a list of products meeting the "Global Fund Mpox Molecular Diagnostic Quality Assurance Standards." Bioperfectus’ Monkeypox Virus Nucleic Acid Detection Kit (Fluorescent PCR method) and Monkeypox Virus and Orthopoxvirus Genus Nucleic Acid Detection Kit (Fluorescent PCR method) have both been included, making Bioperfectus one of only seven companies globally to be listed. As a leading supplier of mpox diagnostic products worldwide, Bioperfectus offers a dual-technology platform based on real-time fluorescence PCR and droplet digital PCR methods, with F3L single-target gene and F3L+B7R dual-target gene/type detection kits. These kits can quickly and accurately identify monkeypox virus from samples such as skin lesion specimens (swabs of rashes, vesicle exudates, vesicle fluid, scabs), throat swabs, whole blood, or serum. Currently, five of Bioperfectus’ detection kits have received CE certification in the EU and MHRA certification in the UK.
Sinocare H1 Net Profit Rise 12.61%

In the first half of 2024, Sinocare (SZ 300298) achieved total revenue of CNY 2.133 billion, reflecting a 6.26% year-on-year increase. Net profit attributable to shareholders reached CNY 1.97 billion, marking a 12.61% year-on-year growth. However, net profit excluding non-recurring items was CNY 1.78 billion, a decrease of 10.38% compared to the same period last year. Sinocare has performed exceptionally well in the Chinese IVD industry, ranking among the top ten companies in terms of both revenue and net profit according to IVD news. Overall, the company has shown steady growth. Sinocare has become the leader in China's blood glucose monitoring retail market, commanding nearly 50% market share and serving over 22 million users. It collaborates with more than 4,000 distributors and nearly 600 pharmacy chains.
Amoy Diagnostics' FGFR2 Gene Breakage Detection Kit Approved in Japan as Companion Diagnostic for Eisai's Tasurgratinib

On September 5, 2024—Amoy Diagnostics, a leading company in tumor companion diagnostics, has announced that its self-developed FGFR2 Gene Breakage Detection Kit (Fluorescence In Situ Hybridization method) has been approved in Japan. This kit will serve as a companion diagnostic for Eisai's innovative targeted drug, Tasurgratinib. The successful approval of this companion diagnostic in Japan marks another significant milestone in precision oncology, offering a new, precise diagnostic and treatment option for patients with cholangiocarcinoma carrying FGFR2 gene fusions.
Haier Biomedical Signs Memorandum of Cooperation with Togo, Promoting the Construction of a China-Africa Health Community

On September 3, President Faure Gnassingbé of the Republic of Togo visited Haier Biomedical, where he toured the company’s smart laboratory, smart blood management, smart public health, and digital hospital solutions. Both sides discussed deepening cooperation in healthcare fields such as sample storage, vaccination, and blood management, culminating in the signing of a strategic cooperation memorandum at Haier Group. Previously, Haier Biomedical had actively participated in multiple projects in Togo, such as healthcare and laboratory infrastructure, basic immunization, and COVID-19 pandemic prevention and control, through partnerships with the Togolese Ministry of Health, National Institute of Health, public hospitals, universities, and UNICEF. The company’s integrated solutions of hardware and software have contributed to strengthening Togo’s basic healthcare capacity.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
