Original from: bioMérieux
· 7.3% organic sales growth in the first nine months of the year, fully in line with annual guidance, with sales reaching a total of €2,992m, primarily driven by a 9% organic increase across the four growth drivers of the GO•28 strategic plan:
- BIOFIRE®1 non-respiratory panels recorded a 10% organic sales growth, driven by increase in all panels and a sustained installed base expansion;
- SPOTFIRE®2 continued its strong growth trajectory with 114% organic sales increase year on year. The pace of instrument installations accelerated in Q3, with over 900 new instruments, bringing the total installed base to more than 5,500 instruments at the end of September more than doubling over 12 months (+160%);
- Microbiology organic sales growth reached 5% excluding China, while the overall performance (+3%) was impacted by a double-digit decline in China;
- Industrial Applications: strong dynamic with a 10% organic sales increase, driven by 15% organic sales growth in the Pharma Quality Control segment leveraging on new products launches (molecular range, 3P® ENTERPRISE);
· BIOFIRE® respiratory panels sales rose by 6% organically, reflecting a strong epidemiological activity in Q1 and lower levels in Q2 and Q3.
· 3% organic sales growth in Q3 impacted by continuous mid-teens decrease in China and an 8% decline in BIOFIRE® Respiratory Panels (low epidemiology). Excluding those two elements, Q3 performance stands at +7.6%.
· 2025 full year guidance:
- Sales are now expected to grow between +5.5% and +6.5% at constant exchange rates (versus between +6% and +7.5% previously) on a late respiratory season.
- Confirmation of the expected +12% to +18% growth in contributive operating income before non-recurring items (CEBIT)3 at constant exchange rates, based on a nine months profitability fully aligned with the GO•28 trajectory.
- The currency effect3 is expected to have a negative impact in the range of around €30m on the 2025 annual CEBIT versus around -€25m previously.
Pierre Boulud, Chief Executive Officer, said: “In a context of low epidemiological activity, we continue to expand our installed base, particularly for BIOFIRE® and SPOTFIRE®, at a faster pace than in 2024, building a strong platform for growth for the coming years. At the same time, bioMérieux continues to deliver on its GO•28 ambition, with significant profitability improvement and sustained innovation through several product launches since the start of the year.”
bioMérieux, a world leader in the field of in vitro diagnostics, today releases its business review for the nine months ended September 30, 2025.
SALES
Consolidated sales amounted to €2,992 million for the first nine months of 2025 versus €2,871 million for the prior-year period, representing a growth of +4.2% as reported. Organic growth (at constant exchange rates and scope of consolidation) reached +7.3% for the first nine months of the year. The currency effect had a negative €85 million impact on sales over 9 months, due to the appreciation of the euro against most of the currencies and notably the US dollar, the Argentinian peso, the Mexican peso and the Turkish lira.

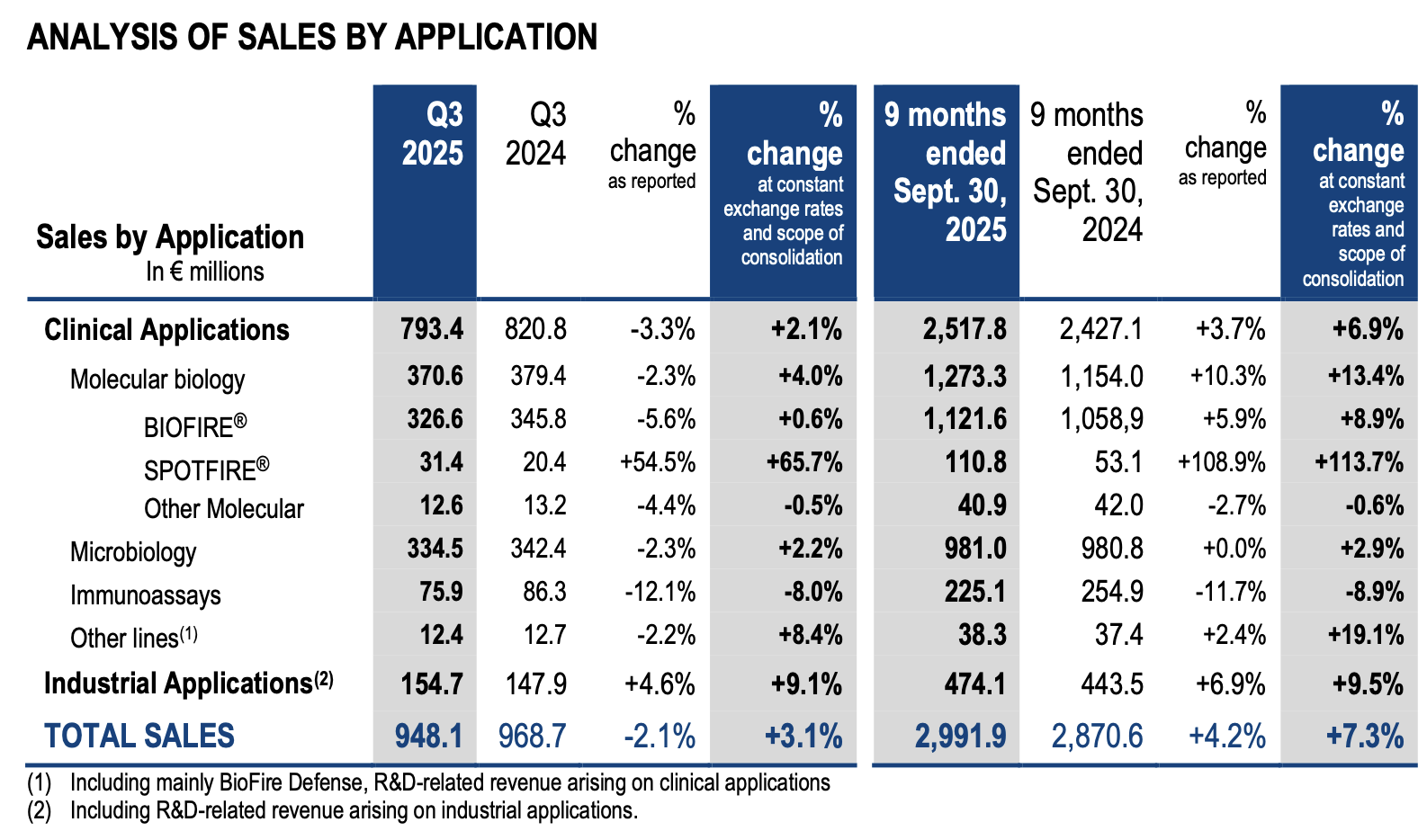
Q3 25 vs Q3 24 like-for-like trends per applications:
· Clinical Applications sales (84% of total sales), increased by 2% over the quarter:
In molecular biology:
- BIOFIRE® non-respiratory panels sales increased by 9%, supported by solid growth in EMEA (Europe, Middle East, Africa) and Latin America, while the US and Asia Pacific performances were slower due to flat sales of the Pneumonia panel, reflecting moderate respiratory activity over the quarter;
- BIOFIRE® respiratory panels sales declined 8%, impacted by a lower circulation of respiratory pathogens compared to the same quarter in 2024;
- Continued BIOFIRE® installed base expansion over the quarter, largely above the number of net installations in Q3 2024;
- SPOTFIRE® sales amounted to €31m in the quarter, an increase of +66% in the context of a low respiratory epidemiology. More than 900 instruments were installed in the quarter, a significant acceleration compared to Q3 24 (600 installations), leading to a total installed base of more than 5,500 instruments at the end of September 2025 (+160% over the last 12 months).
In microbiology, sales increased by 2% impacted by a decline in China due to an unfavorable local healthcare spending trend. Excluding China, microbiology grew by 4% over the quarter on a high year- on-year comparison basis, with reagent sales up 12% in Q3 2024.
In immunoassays, sales declined by 8%, under the combined effect of the drop in the Chinese market, impacted by the implementation of the local volume-based procurement policy, and the continuous decline in global VIDAS® PCT sales (17% of total immunoassays sales). The rest of the VIDAS® business was stable.
· Industrial Applications sales (16% of total sales), delivered a solid organic growth of over 9%, driven primarily by the Pharma Quality Control segment up +15%. This performance was notably supported by a strong momentum in recently launched products in the cytometry and molecular ranges.
ANALYSIS OF SALES BY REGION
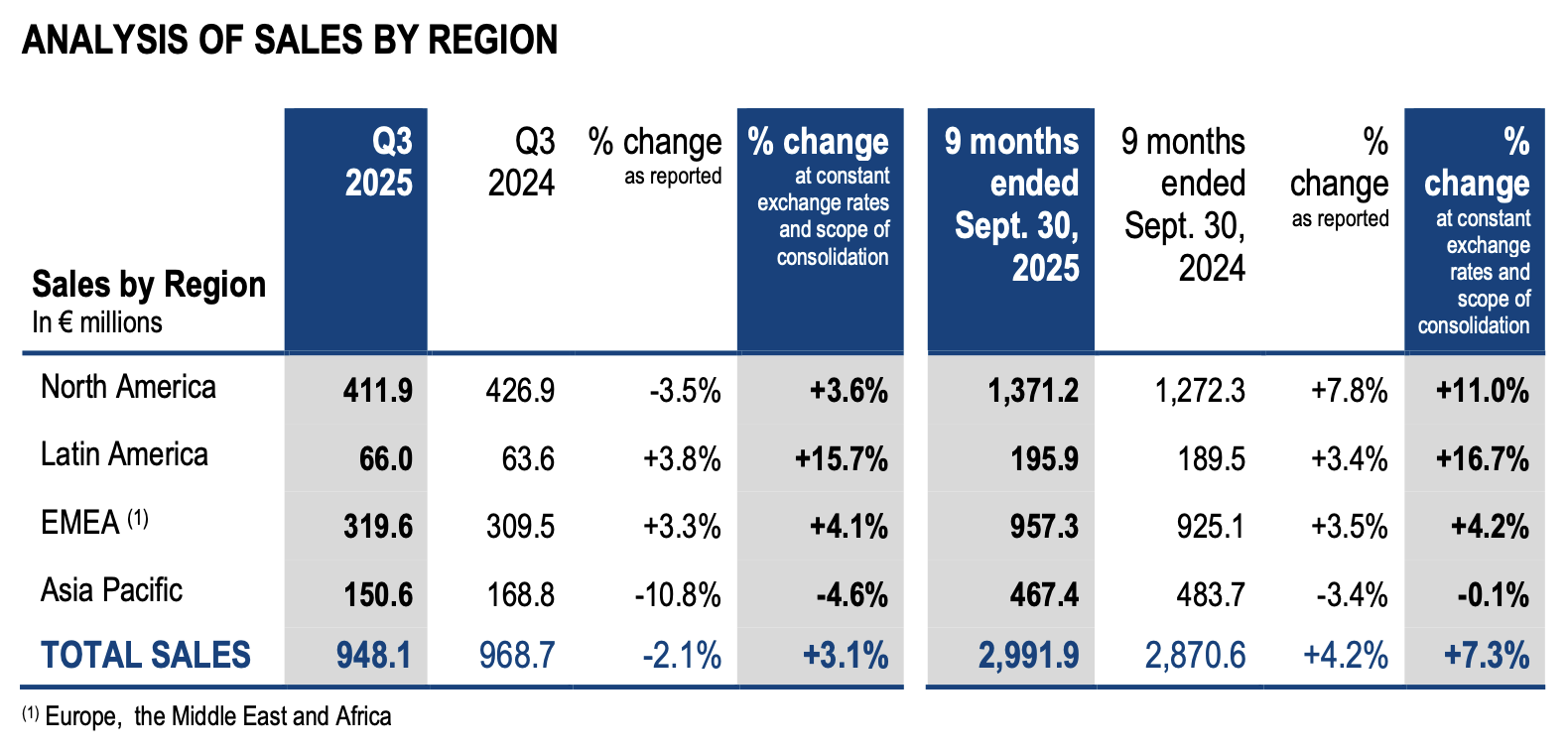
Q3 25 vs Q3 24 like-for-like trends per region:
· In North America (43% of the consolidated total), revenue grew by 4%, driven by a double-digit growth in industrial applications and a 6% increase in microbiology. This performance was partly offset by a decline in BIOFIRE® respiratory panels sales, in a context of low epidemiological activity.
· In Latin America (7% of the consolidated total), the quarterly growth reached 16% (+11% excluding Argentina – hyperinflation country), thanks to a strong momentum in BIOFIRE® panels sales and a double-digit growth in microbiology and industrial applications.
· Sales in the Europe – Middle East – Africa region (34% of the consolidated total) came to €320 million for the quarter, up 4% year-on-year, driven by close to 20% sales growth in BIOFIRE® non respiratory panels and a double digit increase in industrial applications sales while BIOFIRE® respiratory panels sales declined mid-teens as a result of a significantly lower epidemiological activity compared to 2024.
· Sales in the Asia Pacific region (16% of the consolidated total) amounted to €151 million for the third quarter of 2025, down 5% from the prior-year period. Excluding China, sales were up 4%, led by the success of SPOTFIRE® in Japan and a double-digit sales growth of BIOFIRE® non-respiratory panels while BIOFIRE® respiratory panels sales were significantly down.
EVENTS OF THIRD-QUARTER 2025
· bioMérieux receives U.S. 510(k) clearance and CLIA-waiver for Anterior Nasal Swab specimens, an additional sample type for use with the BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) Panel Mini
On August 18, 2025, bioMérieux announced that its BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) Panel Mini has received U.S. Food and Drug Administration (FDA) 510(k) clearance and Clinical Laboratory Improvement Amendments (CLIA) waiver for the addition of Anterior Nasal Swab (ANS) as a validated specimen type for this panel, specifically for use with the respiratory test menu. By swabbing only the anterior part of the nasal cavity, ANS provides significantly more comfort for the patient.
· bioMérieux develops game changing Quality Control solution GENE-UP® PRO HRM
On August 5, 2025, bioMérieux announced the launch of GENE-UP® PRO HRM—the first DNA-based test commercially developed to detect heat-resistant molds at the molecular level. Developed in partnership with historic brand Ocean Spray Cranberries, Inc., the game-changing testing innovation can accurately identify the presence of viable heat-resistant molds and provides a significantly shorter time-to-result, reducing the current Compendial Method 15-day result time down to only 72 hours.
· bioMérieux recognized for Best System Performance and Best Service with 2025 IMV ServiceTrak™ Clinical Laboratory Awards
On August 5, 2025, bioMérieux received two 2025 IMV ServiceTrak™ Clinical Laboratory Awards for excellence in Immunoassay. These awards highlight bioMérieux’s continued commitment to delivering industry-leading diagnostic solutions and exceptional customer support to clinical laboratories.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
