Original from AHA News
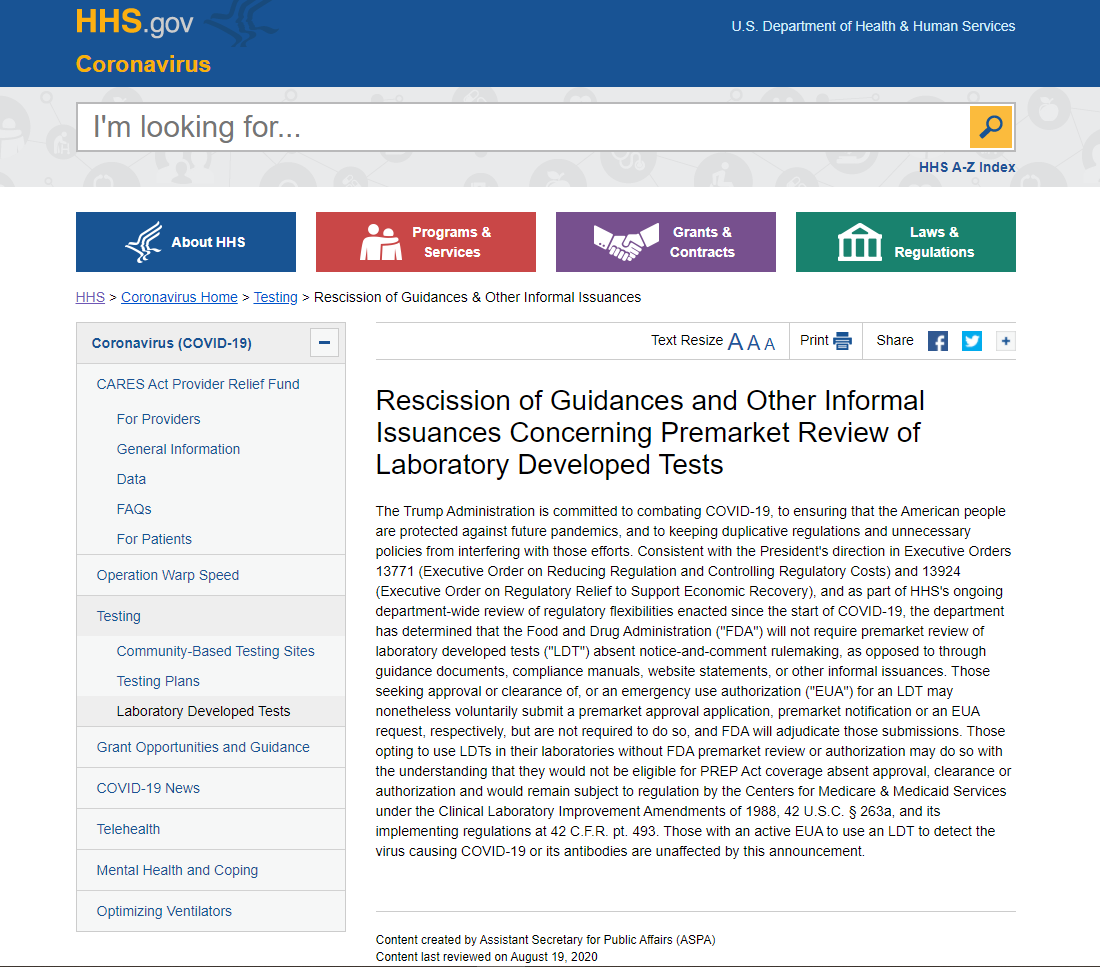
In an effort to reduce regulatory burden, the Food and Drug Administration will not require developers to submit a premarket approval application, premarket notification or emergency use authorization for laboratory developed tests, the Department of Health and Human Services announced on August 19th.
Laboratories opting to use these tests would not be eligible for Public Readiness and Emergency Preparedness Act coverage and would remain subject to regulation by the Centers for Medicare & Medicaid Services under the Clinical Laboratory Improvement Amendments of 1988 and its implementing regulations, HHS said. LDT developers may voluntarily apply for approval, clearance or an EUA, and FDA will adjudicate those submissions.
LDTs are developed, validated and performed by individual laboratories, including hospital laboratories, when commercial diagnostic tests do not exist or meet clinical needs. AHA has repeatedly urged the agency not to regulate LDTs as medical devices, which would reduce patient access to many critical tests and hinder technological and clinical innovation.
Picture from HHS Official Website
FAQs on Laboratory Developed Tests (LDTs)



Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
