


Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

A new UK industry body designed to help medical device regulators navigate the new legal landscape of a post-Brexit Britain has launched with backing from 11 companies.

The US Food and Drug Administration on Tuesday issued a letter to medical device manufacturers and sponsors of device studies warning them to independently verify performance test results before submitting them to the agency due to concerns about fraudulent results.

If Chinese chemiluminescence would eventually replace imported products completely, it must ensure the accuracy and reliability of the test results. In addition to the improvement of equipment system and methodology, the accuracy of results will involve two very core competitions in the future.

Freenome said on Thursday that it raised $254 million in funding from existing and new investors to advance its pipeline of single- and multi-cancer early detection tests.
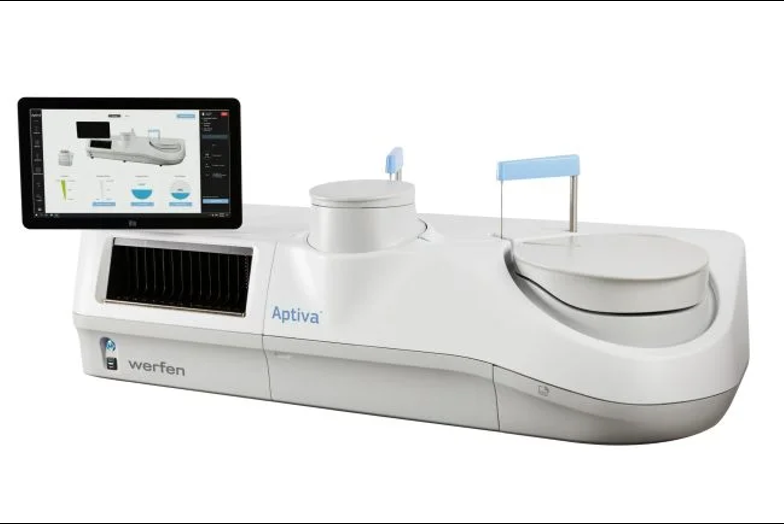
Werfen has received CE marking for its Aptiva antiphospholipid syndrome (APS) Immunoglobulin G (IgG) and Immunoglobulin M (IgM) reagents, meaning the in vitro diagnostics can now be used in the EU.

Bio-Rad Laboratories, Inc. (NYSE: BIO and BIO.B), a global leader in life science research and clinical diagnostics products, today announced financial results for the fourth quarter and full year ended December 31, 2023.

QuidelOrtho Corporation (Nasdaq: QDEL) (the “Company” or “QuidelOrtho”), a global provider of innovative in vitro diagnostic technologies designed for point-of-care settings, clinical labs and transfusion medicine, today announced financial results for the fourth quarter and full-year ended December 31, 2023.

The analyzer will develop in two directions as per different customer needs and application scenarios.

SCIEX, a global leader in life science analytical technologies, launches the Echo® MS+ system at SLAS 2024. The system couples proprietary Acoustic Ejection Mass Spectrometry technology and Open Port Interface (OPI) sampling with the capabilities of either the SCIEX ZenoTOF 7600 or Triple Quad 6500+ system to deliver precise qualitative and quantitative results, through an expanded panel of robust high-throughput screening workflows.

BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced results for its first quarter of fiscal 2024, which ended December 31, 2023.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
