


Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

The European Commission (EC) has proposed an extension of the transition period for the IVDR, aiming to maintain the availability of essential healthcare products and ensure patient care.
China's medical device industry has grown at a compound annual growth rate of 10.54% over the past five years and has become the second largest market in the world.

New research suggests a combination of molecular profiling on tumor tissue and corresponding circulating tumor DNA (ctDNA) samples can provide clinically actionable clues that may be missed using either approach alone, particularly for individuals with breast cancer or non-small cell lung cancer (NSCLC).
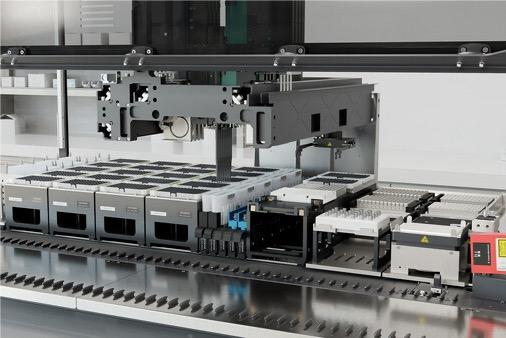
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, announced a collaboration agreement with Hamilton, a leading global manufacturer of laboratory automation technology, to develop automated applications together with robotics-compatible reagent kits to enable greater standardization and reduced human error when conducting large-scale single-cell multiomics experiments.

Analysis of molecules that travel from the brain into the blood could enable noninvasive monitoring of gene expression in the organ, according to research published in Nature Biotechnology.

Invitae (NYSE: NVTA), a leading medical genetics company, today announced it has completed the sale of certain reproductive health assets, which include carrier screening and non-invasive prenatal screening, to Natera (NASDAQ: NTRA).

Myriad Genetics said Thursday that it has entered into a definitive agreement to acquire select assets from Intermountain Precision Genomics' laboratory business, including the Precise Tumor Test, the Precise Liquid Test, and IPG’s CLIA-certified laboratory in St. George, Utah.

Fujirebio Holdings, Inc. and Agappe Diagnostics Ltd today announced that they have entered into an agreement of Contract Development and Manufacturing Organization (CDMO) partnership for Cartridge based CLIA system reagents manufacturing project for the immunology equipment Mispa i60 and Mispa i121. Notably, the analyzers and reagents will be sold under Agappe’s brand, making Agappe the first Indian local company with a complete Chemiluminescence Solution comprising locally manufactured reagents.

Dian Diagnostics Group is teaming up with the Gulf Medical University in the United Arab Emirates on providing testing lab services and a number of other fields, as part of the Chinese health testing service provider’s strategy to expand its global footprint.

Cepheid announced today that it has received FDA Clearance with Clinical Laboratory Improvement Amendments (CLIA) waiver for Xpert® Xpress MVP. This multiplex vaginal panel can now be performed in near-patient settings, enabling results within 60 minutes from a single specimen for Bacterial Vaginosis (BV), Vulvovaginal Candidiasis (VVC), and Trichomoniasis (TV). This expanded claim exemplifies Cepheid's commitment to providing expanded access to women's and sexual health by making PCR testing accessible at the point of care. The test runs on Cepheid's GeneXpert Xpress instruments and has been approved for testing women fourteen years of age and older.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
