


Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Agilent Technologies Inc. (NYSE: A) today announced a new organizational structure to accelerate the company's operational transformation to drive higher growth through a market-focused, customer-centric enterprise strategy. The new structure, including new leadership roles, takes effect immediately.

Agilent Technologies Inc. (NYSE: A) today reported revenue of $1.70 billion for the fourth quarter ended October 31, 2024, an increase of 0.8% reported and a decline of 0.3% core(1) compared to the fourth quarter of 2023.

With a large number of tumor-targeted drugs on the market, companion diagnosis is increasingly used in the process of tumor treatment. The results of using tissue samples for tumor companion diagnosis are more accurate than selecting plasma-free DNA (cell-free DNA, cfDNA).

Xcell Therapeutics is expanding the business scope of its exosome isolation and purification device, “EXODUS,” into the diagnostics market.

Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today the U.S. Food and Drug Administration (FDA) approval of a label expansion into biliary tract cancer (BTC) for the PATHWAY® anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody* test. This test is now the first and only FDA-approved companion diagnostic to aid in the assessment of HER2-positive status to identify BTC patients who are eligible for treatment with Jazz Pharmaceuticals' ZIIHERA® (zanidatamab-hrii).

Artificial intelligence continues to reshape healthcare, but a new study highlights the challenges of integrating AI tools into medical practice. ChatGPT-4, an AI chatbot developed by OpenAI, outperformed doctors in a diagnostic accuracy study, raising questions about how effectively physicians can use such technology.
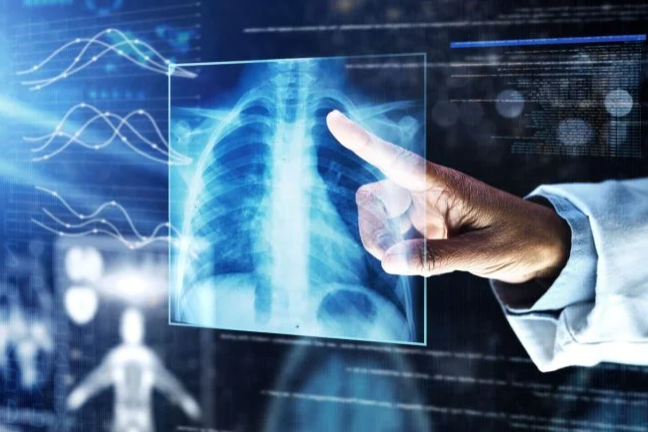
Sheba Medical Center’s innovation arm, Accelerate, Redesign, and Collaborate (ARC), has partnered with Roche to advance the diagnosis and treatment of non-small cell lung cancer (NSCLC) by leveraging AI.

Li Li, commissioner of China's National Medical Products Administration (NMPA), led a delegation to Vietnam and Thailand from 11 to 15 November to enhance bilateral collaboration in pharmaceutical regulation. During the visit, Li signed memorandums of understanding (MoUs) with health authorities in both countries.

Myriad Genetics, Inc. (NASDAQ: MYGN), a leader in genetic testing and precision medicine, announced updates to its agreement with Illumina Inc. (NASDAQ: ILMN), a global leader in DNA sequencing and array-based technologies.

Tagomics Ltd., a pioneering biomarker discovery and diagnostics company, today announced it has entered into a strategic co-marketing agreement with Agilent Technologies, a leading tools provider in the genomics sector. The agreement offers select customers the opportunity to access Tagomics’ and Agilent Technologies’ combined innovative offerings through an exclusive technology access program hosted out of Tagomics’ service lab in Cambridge.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
