
The 8th Experimental Medicine Conference of China is scheduled to be held at Primus Hotel Nanchang International Expo City on 27 May in 2023.

On 13 March, BioMérieux China signed a strategic cooperation agreement with Accunome Biotechnology Co., Ltd.

Agilent Technologies announced a multiyear distribution agreement with Proscia to offer a comprehensive digital diagnostic pathology system.

QIAGEN announced it has entered into a strategic partnership with Servier, a global pharmaceutical group, to develop a companion diagnostic test for TIBSOVO®, an isocitrate dehydrogenase-1 (IDH1) inhibitor indicated for the treatment of the blood cancer acute myeloid leukemia (AML).

Roche announced on Monday that its Ventana PD-L1 (SP263) assay has received approval from the US Food and Drug Administration for use as a companion diagnostic with Regeneron's Libtayo (cemiplimab) in lung cancer patients.
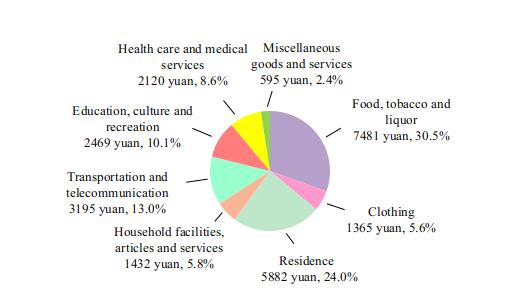
By the end of 2022, there were 1,033,000 medical and health institutions in China, including 37,000 hospitals. The medical and health institutions in China possessed 9.75 million beds, of which hospitals possessed 7.66 million and township health centers had 1.45 million.
✔ All (16)
✔ Press release (2)
✔ Industry news (14)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
