
In the face of the sudden monkeypox virus epidemic, many Chinese in vitro diagnostic enterprises responded quickly, completed the R & D and launched the monkeypox virus nucleic acid detection kit at the first time, effectively helping overseas to carry out the monkeypox virus prevention.

On May 10, the National Development and Reform Commission issued the "14th Five-Year Plan for Bio-economic Development" (hereinafter referred to as the "Plan"), which is also the first top-level design in China's bio-economic field.
A point-of-care COVID-19 test developed by researchers at the University of Illinois Urbana-Champaign can now detect and differentiate the alpha variant of the SARS-CoV-2 virus from earlier strains in saliva samples.

BioMérieux on Tuesday announced it has entered into an agreement to acquire Specific Diagnostics for an acquisition price equivalent to 3.3 percent of BioMérieux's market capitalization as of April 11, paid with a combination of cash and shares issued to Specific Diagnostics shareholders.

On April 13, National Medical Products Administrations (NMPA) reviewed and approved Zhuhai Livzon Diagnostics Inc. and Shanghai BioGerm Medical Technology Co., Ltd's COVID-19 Antigen Test.

The US Food and Drug Administration this week granted separate Emergency Use Authorizations for over-the-counter SARS-CoV-2 antigen self-tests developed by Osang Healthcare.
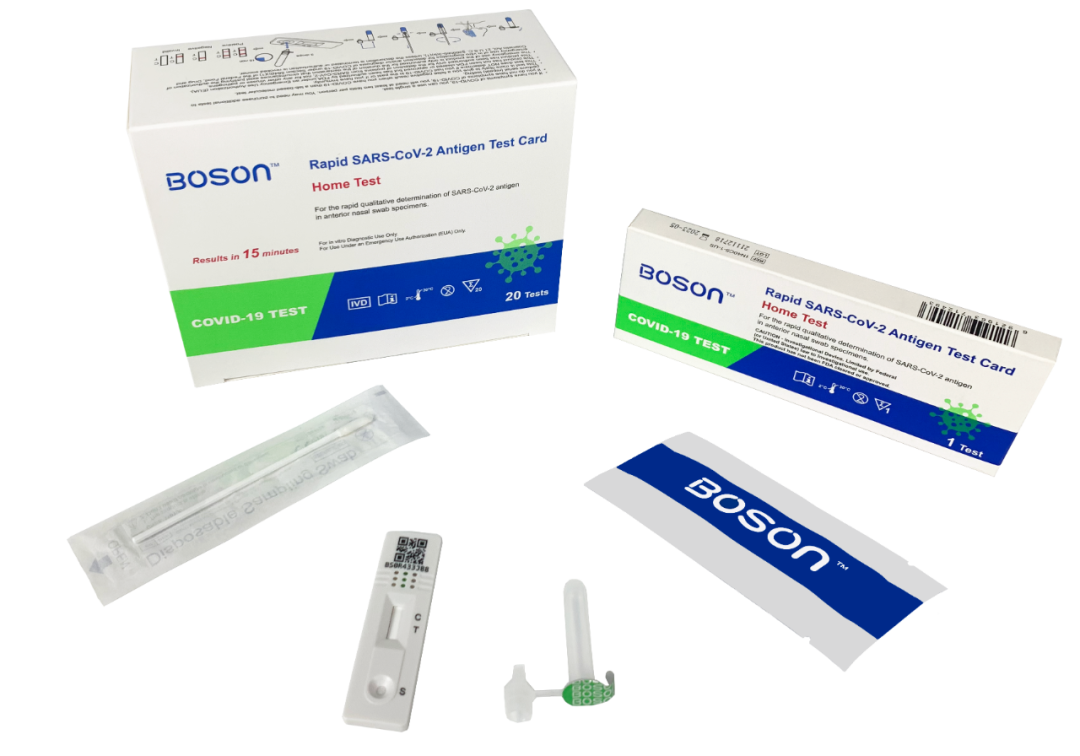
On April 6, it was reported that the Rapid SARS-CoV-2 Antigen Test Card produced by Xiamen Boson Biotech Co., Ltd. has obtained the FDA Emergency Use Authorization (EUA).

Visby Medical announced on Friday that it will execute a $25.5 million contract option with the Office of the Assistant Secretary for Preparedness and Response to develop an at-home version of its handheld, PCR-based test for influenza and COVID-19.
Genetic testing of saliva samples identifies the SARS-CoV-2 virus more quickly than testing of nasal swabs. The research is published March 21 in Microbiology Spectrum, a journal of the American Society for Microbiology.

The US Food and Drug Administration last week granted separate Emergency Use Authorizations for two SARS-CoV-2 antigen tests developed by Siemens Healthineers.
✔ All (81)
✔ Press release (1)
✔ Industry news (80)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
