
Lunit on Monday said it has started a collaboration with Daiichi Sankyo to use its artificial intelligence-based digital pathology tools to advance translational research and biomarker discovery for two of Daiichi's oncology pipeline programs.
New research suggests that individuals who consistently carry Staphylococcus aureusin their noses have other nasal microbial community features that are distinct from those found in individuals without persistent S. aureus colonization.

Circular Genomics announced Monday that it raised $15 million in a Series A financing round led by Mountain Group Partners.

Lunit (KRX:328130.KQ), a leading provider of AI for cancer diagnostics and therapeutics, and Agilent Technologies Inc. (NYSE: A), a global leader in life sciences, diagnostics, and applied chemical markets, today announced a nonexclusive collaboration to develop AI-based companion diagnostic solutions. The collaboration will leverage Lunit's AI technology and Agilent's expertise in tissue-based companion diagnostics to create advanced solutions that meet the demand of novel and complex biomarker assays in drug development.
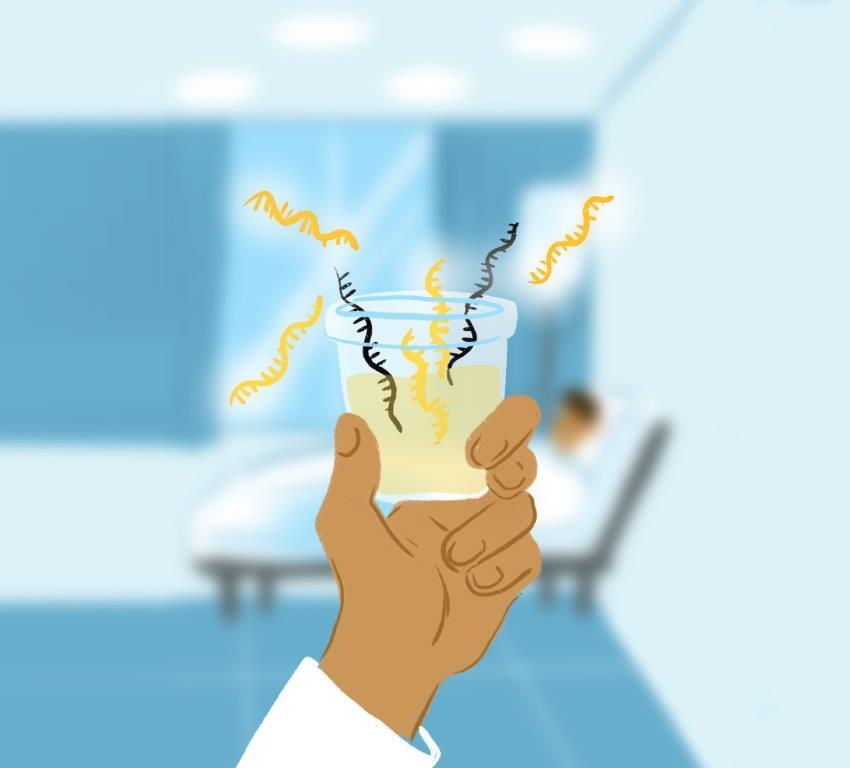
Researchers at the Johns Hopkins Kimmel Cancer Center, Johns Hopkins All Children’s Hospital and four other institutions have devised a novel method to test for prostate cancer using biomarkers present in urine, funded in part by the National Institutes of Health. This approach could significantly reduce the need for invasive, often painful biopsies, they say.

Quanterix Corporation (NASDAQ: QTRX), a company fueling scientific discovery through ultrasensitive biomarker detection, today announced the New York State Department of Health (NYSDOH) has granted the Quanterix Accelerator Laboratory a clinical laboratory permit in the Clinical Chemistry category. The permit approval expands advanced biomarker research led by Quanterix, deepening collaborations with clinical, pharmaceutical, and research partners. The Quanterix Accelerator Lab is now fully CLIA-certified in all 50 states, enabling comprehensive clinical testing and biomarker analysis nationwide.

Currently, most level-Ⅲ hospitals and some level-Ⅱ hospitals in China are equipped with automated and intelligent diagnostic devices.

Augurex Life Sciences Corp., a leader in autoimmune biomarker-based diagnostics, today announced that Health Canada has approved SPINEstat™, a first-in-class diagnostic blood test for axial spondyloarthritis (axSpA), as a Class II medical device. By detecting auto-antibodies to the 14-3-3eta protein, SPINEstat™ provides an important new clinically validated and objective biomarker to aid in the early and accurate diagnosis of axSpA.

The National Medical Products Administration (NMPA) has announced the priority review approval for Roche Diagnostics GmbH under its Priority Approval Procedure for Medical Devices (2016 No. 168).

Fujirebio Holdings, Inc. (President & CEO: Goki Ishikawa; Head Office: Minato-ku, Tokyo), a wholly-owned subsidiary of H.U. Group Holdings, Inc. (Chairman, President and Group CEO: Shigekazu Takeuchi; Head Office: Minato-ku, Tokyo), and Eisai Co., Ltd. (Representative Corporate Officer and CEO: Haruo Naito; Head Office: Bunkyo-ku, Tokyo; hereinafter "Eisai") announced today that they have entered into a memorandum of understanding for the joint research and social implementation of novel blood-based biomarkers in the field of neurodegenerative diseases.
✔ All (46)
✔ Press release (0)
✔ Industry news (46)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
