
Thermo Fisher Scientific said Tuesday that it has signed a memorandum of understanding with Singapore’s National University Hospital (NUH) and Singapore RNA technology company Mirxes to develop and validate next-generation sequencing assays for early cancer detection in the country.

QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) today announced the release of a new version of its clinical decision support software, QIAGEN Clinical Insight Interpret (QCI Interpret), that brings significant performance and scalability enhancements tailored for high-throughput, next-generation sequencing (NGS) labs moving to larger test panels and higher test volumes. The latest version of QCI Interpret introduces improvements that accelerate critical lab performance criteria for turn-around-time, diagnostic yield and quality results.

Burning Rock Biotech Limited (NASDAQ: BNR and LSE: BNR, the “Company” or “Burning Rock”) has recently announced a collaboration with Bayer to develop next-generation sequencing (NGS)-based companion diagnostic assays (CDx), aiming to provide diagnostic methods, to enable treatment choice for patients with cancer in China, while driving innovation and development in cancer therapy. This collaboration will focus on the development of companion diagnostic products in China, jointly developing NGS-based CDx for Bayer's growing portfolio of precision cancer therapies.

QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) and Myriad Genetics (NASDAQ: MYGN) today announced they will develop a globally distributable kit-based test for analyzing Homologous Recombination Deficiency (HRD) status. This next-generation sequencing (NGS) test aims to support research into personalized medicine in multiple solid tumor types, including ovarian cancer and is expected to enhance decentralized testing capacities once a regulated product is developed with pharmaceutical partners. The project builds on the recently announced master collaboration agreement between the two companies.

Oxford Nanopore Technologies has launched a new Pharmacogenomics (PGx) Beta Program with Twist Biosciences.

QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) today announced an updated version of its clinical decision support platform, QIAGEN Clinical Insight Interpret for NGS molecular profiling of hereditary and somatic diseases, has received the European Union (EU) Technical Documentation Assessment and Quality Management System certificate under the European In Vitro Diagnostic Medical Device Regulation (EU) 2017/746 (IVDR).

QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) today announced the launch of the QIAseq xHYB Mycobacterium tuberculosis Panel for research use, a new tool in the fight against tuberculosis (TB), the world’s leading infectious disease killer.

Oxford Nanopore Technologies has signed a partnership with artificial intelligence developer SeqOne to support its next-generation sequencing approach in clinical diagnostic testing.

Burning Rock Biotech Limited (NASDAQ: BNR and LSE: BNR, the “Company” or “Burning Rock”), a company focused on the application of next generation sequencing (NGS) technology in the field of precision oncology, today reported financial results for the three months and the year ended December 31, 2023.
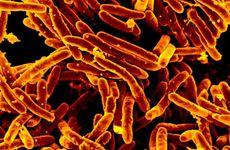
The World Health Organization has published updated guidelines for the rapid diagnosis of tuberculosis, including new recommendations on the use of targeted next-generation sequencing (NGS) tests for drug-resistant TB.
✔ All (82)
✔ Press release (0)
✔ Industry news (82)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
