
Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that it has received CE mark for its Elecsys® Dengue Ag test – a high-throughput, fully automated immunoassay to be used as an aid in the diagnosis of an acute dengue virus infection. This milestone promises to set a new standard for efficiency and reliability in tackling the growing global challenge of dengue fever.
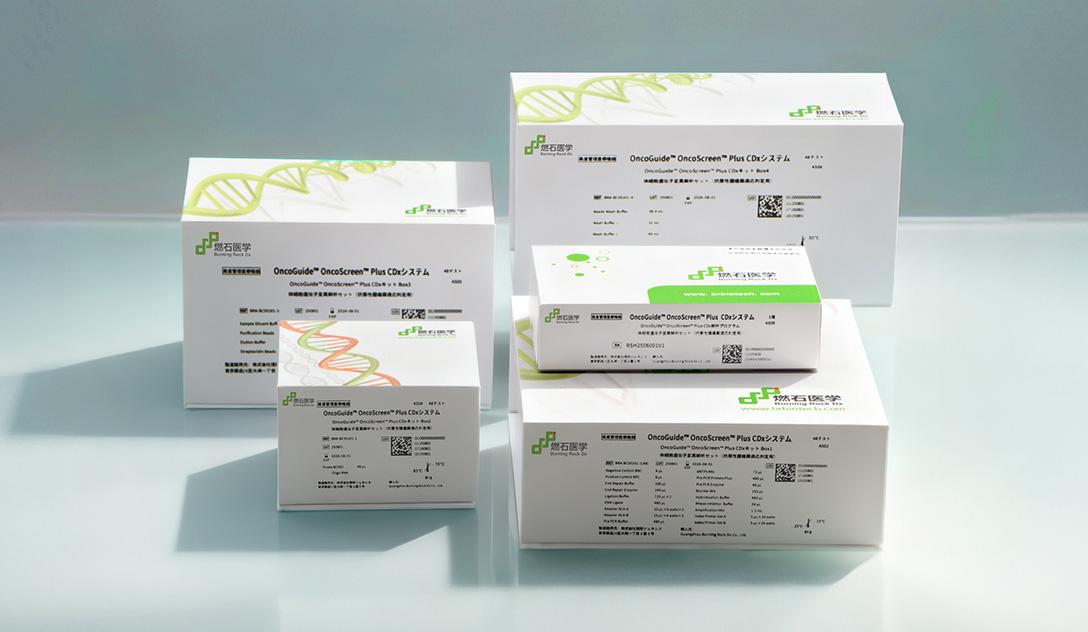
Riken Genesis Co., Ltd. (Riken Genesis) and Burning Rock Biotech Limited (NASDAQ: BNR, “Burning Rock”) today announced that the OncoGuide™ OncoScreen™ Plus CDx System based on OncoScreen™ Plus to be used as a companion diagnostic for AstraZeneca’s capivasertib has received Manufacturing and Marketing Approval from Japan’s Ministry of Health, Labour and Welfare (MHLW).

MedMira Inc. (MedMira) (TSXV: MIR) announced today that it has received approval from Health Canada for its Reveal® TP (Syphilis) Antibody Test (Reveal® TP) – the fastest standalone screening device for syphilis in Canada.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Illumina, Inc. (NASDAQ: ILMN), a global leader in DNA sequencing and array-based technologies, announced today that it has received approval from the Ministry of Health, Labour and Welfare (MHLW) for TruSightTM Oncology (TSO) Comprehensive for Class III/IV Medical Device (Specially Controlled Medical Device) in Japan.

The US Food and Drug Administration (FDA) has set an “aggressive” timeline to implement generative artificial intelligence (genAI) across the agency by 30 June 2025.

Augurex Life Sciences Corp., a leader in autoimmune biomarker-based diagnostics, today announced that Health Canada has approved SPINEstat™, a first-in-class diagnostic blood test for axial spondyloarthritis (axSpA), as a Class II medical device. By detecting auto-antibodies to the 14-3-3eta protein, SPINEstat™ provides an important new clinically validated and objective biomarker to aid in the early and accurate diagnosis of axSpA.

QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) today announced that the U.S. Food and Drug Administration (FDA) has cleared the QIAstat-Dx Gastrointestinal Panel 2 Mini B for clinical use, further strengthening its syndromic testing portfolio in the United States.
✔ All (29)
✔ Press release (0)
✔ Industry news (29)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
