Blood typing-related products mainly include two sectors of blood typing instruments and blood typing reagents.
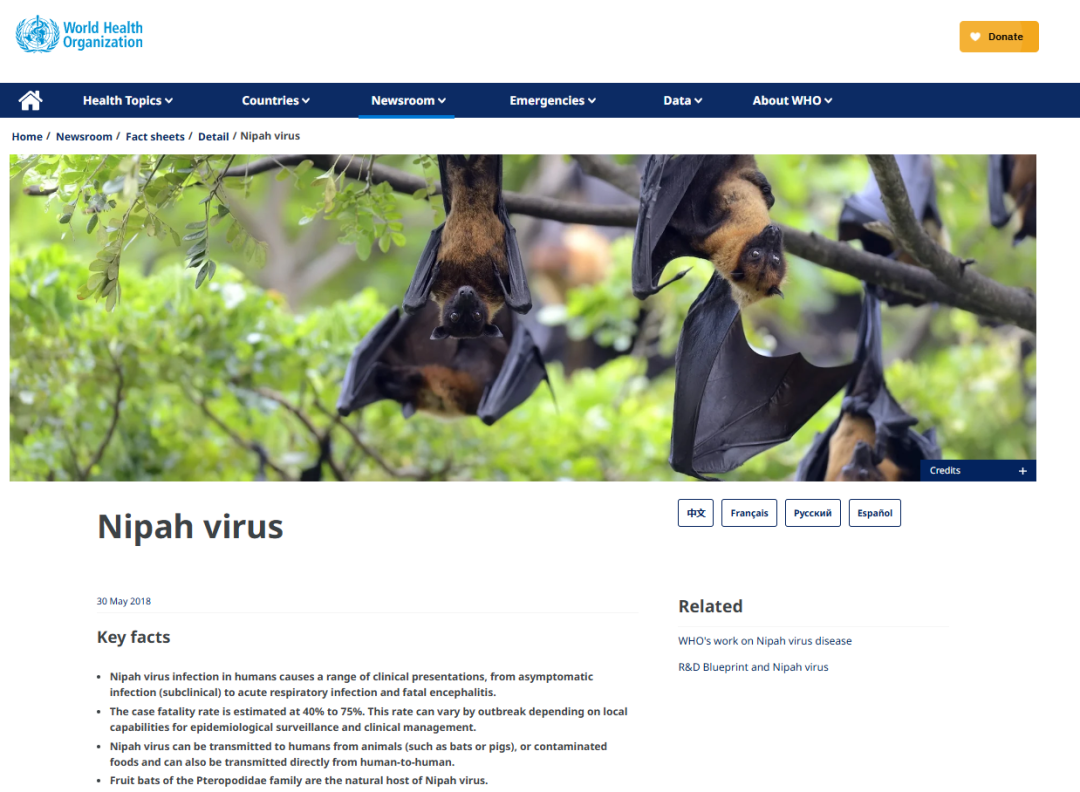
Recently, an outbreak of Nipah virus occurred in West Bengal, India, with 5 confirmed cases reported, including several healthcare workers. Nearly 100 people have been placed under home quarantine, and one patient is in critical condition, highlighting the urgency of epidemic control.

Synthego, a recognized leader in CRISPR solutions, announces its formal entry into the molecular and clinical diagnostic reagents sector. Building upon its reputation for rigorous quality, custom manufacturing, and innovation, Synthego's significant expansion of its product portfolio empowers researchers, clinicians, and diagnostic laboratories with high-quality, reliable reagents designed to accelerate the development and deployment of cutting-edge diagnostic tests.

Systemic lupus erythematosus (SLE) is the most common systemic autoimmune disease in China, with an incidence of 30.13–70.41/100000 population. The kidney is the most commonly involved organ in SLE, and 40% ~ 60% of SLE patients have lupus nephritis (LN).

In vitro fertilization embryos are prone to chromosomal aneuploidy abnormalities. Chromosome aneuploidy refers to the increase or decrease in the number of a single chromosome or multiple chromosomes.

High-throughput sequencing (HTS) has started to be applied in tumor companion diagnosis in recent years.

Werfen today announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the Aptiva® Antiphospholipid Syndrome (APS) Immunoglobulin G (IgG) and Immunoglobulin M (IgM) Reagents.

Lymphoma, also known as malignant lymphoma, is a general term for a group of malignant tumors originating from the lymphopoietic system. It is one of the most common tumors in China. In 2020, there were 544,352 new cases of non-Hodgkin lymphoma (NHL) worldwide, ranking 13th among all new cases of malignancies.

The clinical application of molecular diagnostic technology is mainly in the form of detecting nucleic acid markers, including reagents and instruments, which are regulated by the National Medical Product Administration (hereinafter referred to as NMPA).

At present, the upstream raw materials of biochemical reagents mainly rely on imports. For the long-term development of the industry, the preparation technology of raw materials must be mastered.
✔ All (36)
✔ Press release (0)
✔ Industry news (36)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
