
Siemens Healthineers said on Monday that the firm has received CE marking for a prognostic blood test that the firm plans to launch later this year in Europe to aid the management of multiple sclerosis.

Roche announced this week that its Ventana PD-L1 (SP263) assay received a CE-IVDR label expansion for use as a companion diagnostic with Regeneron's Libtayo (cemiplimab) in combination with platinum-based doublet chemotherapy in lung cancer patients.

Revvity's EUROIMMUN business, a leading provider of high-quality in-vitro diagnostic products, and ALPCO-GeneProof, a global leader in molecular diagnostics, jointly announced a strategic partnership to enhance the availability of GeneProof PCR kits throughout the European Union. This collaboration brings together EUROIMMUN's extensive distribution network and support infrastructure with ALPCO-GeneProof's innovative molecular diagnostic technologies.

Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today the approval of the CE Mark for the VENTANA® HER2 (4B5) Rabbit Monoclonal Primary Antibody RxDx* to identify metastatic breast cancer patients with low HER2 expression for whom ENHERTU® (trastuzumab deruxtecan) may be considered as a targeted treatment. The test, which is branded PATHWAY in the United States, received US Food and Drug Administration (FDA) approval in October 2022. ENHERTU is a specifically engineered HER2-directed antibody drug conjugate (ADC) being jointly developed and commercialised by Daiichi Sankyo and AstraZeneca.

C-luminary Biotech was founded in 2018 and is headquartered in Chengdu, China, with a research and development center in Suzhou and industrial platforms in Sichuan and Hunan, is a professional enterprise engaged in the research, development, production, and sale of in vitro diagnostic products.
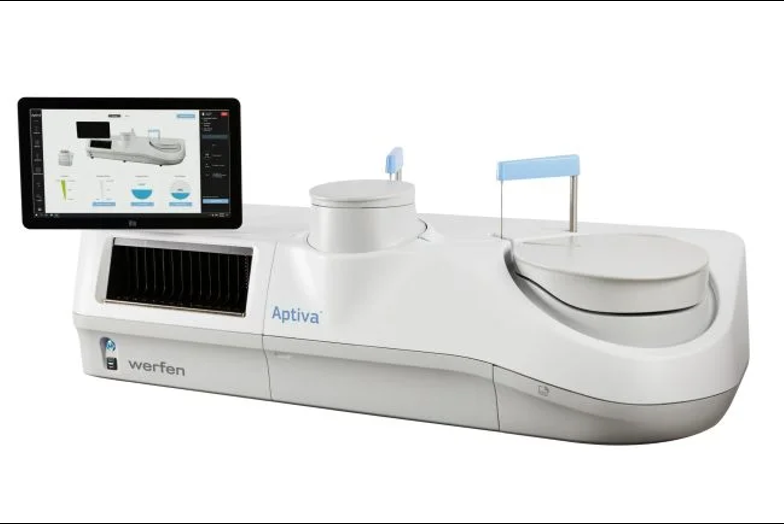
Werfen has received CE marking for its Aptiva antiphospholipid syndrome (APS) Immunoglobulin G (IgG) and Immunoglobulin M (IgM) reagents, meaning the in vitro diagnostics can now be used in the EU.

Roche announced on Tuesday that it has received CE marking for a claim extension on its Elecsys Anti-Müllerian Hormone (AMH) Plus test.

The UK government on Tuesday said it has issued legislation for the implementation of new regulations governing in vitro diagnostics and medical devices.

Devyser Diagnostics AB has entered an in vitro diagnostic development agreement with Illumina, Inc. (Nasdaq: ILMN). The agreement enables Devyser to develop and offer its in vitro diagnostic (IVD) tests on Illumina MiSeqDx next-generation sequencing (NGS) instrument in the United States and Europe.

CACLP's initiative to launch an English/Chinese webinar for non-EU IVD players in next January is set to provide a crucial platform for understanding EU IVDR registration requirements.
✔ All (50)
✔ Press release (0)
✔ Industry news (50)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
