
MSD (tradename of Merck & Co., Inc., Rahway, N.J., USA), today announced the successful completion of the cash tender offer, through a subsidiary, for all the outstanding shares of common stock of Cidara Therapeutics, Inc. (Nasdaq: CDTX) (“Cidara”).

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Merck (NYSE: MRK), known as MSD outside of the United States and Canada, and Cidara Therapeutics, Inc. (Nasdaq: CDTX) (“Cidara”), a biotechnology company developing drug-Fc conjugate (DFC) therapeutics, today announced that the companies have entered into a definitive agreement under which Merck, through a subsidiary, will acquire Cidara for $221.50 per share in cash, for a total transaction value of approximately $9.2 billion.
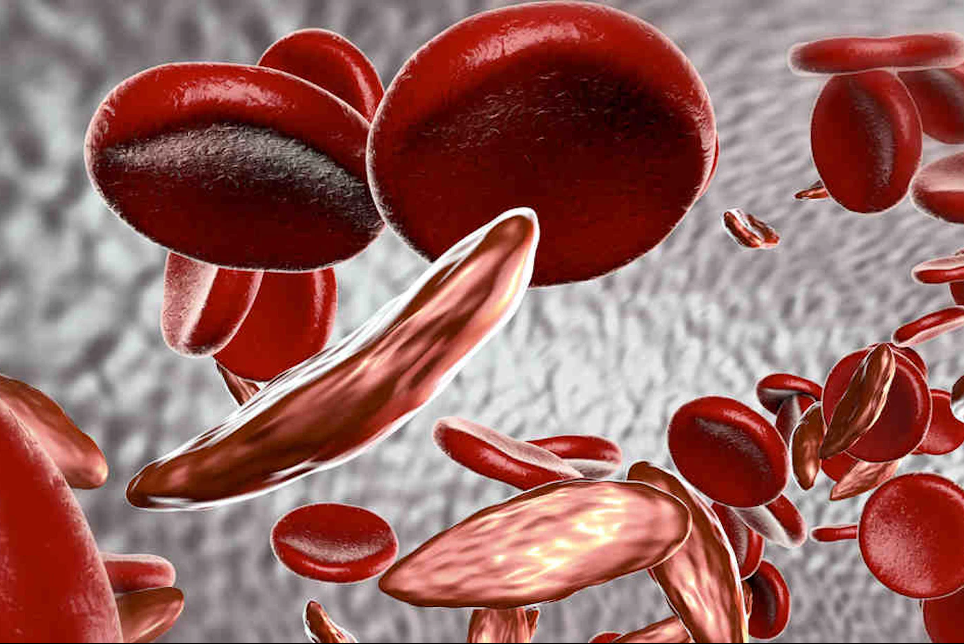
U.K. medical regulators have approved a “world-first” gene therapy that aims to cure two blood disorders: sickle cell disease (SCD) and transfusion-dependent β-thalassemia. They are the first regulatory body to approve the treatment.

Eli Lilly and Company (NYSE: LLY) and Sigilon Therapeutics, Inc. (Nasdaq: SGTX) today announced a definitive agreement for Lilly to acquire Sigilon, a biopharmaceutical company that seeks to develop functional cures for patients with a broad range of acute and chronic diseases.

GenScript Biotech, a preeminent global provider of life sciences services, and Boan Biotech, an integrated biopharmaceutical company specializing in the development, manufacturing and commercialization of biologics, announced a collaboration for the development and production of GenCircleTM dsDNA, a small circular double-stranded DNA vector without resistance markers, a key ingredient in gene and cell therapy.

Alzheimer's disease (AD) is a common neurodegenerative disease that affects the cognitive and memory abilities of the brain.

On August 5, 2021, Pillar Biosciences announced the FDA has given PMA to its oncoReveal™ Dx Lung and Colon Cancer Assay, an NGS tissue-based companion diagnostic test for the detection of somatic mutations in DNA derived from non-small cell lung cancer (NSCLC) and colorectal (CRC) cancer tumors
✔ All (8)
✔ Press release (0)
✔ Industry news (8)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
