
SEKISUI Diagnostics’ microbial CDMO business announces it has completed construction of its £15.7 million ($20.7 million) cGMP capacity expansion at its UK site for clinical-grade drug substance manufacturing. Following appropriate licensure, this expansion will enable manufacturing capabilities for common drug types including enzymes, proteins and antibody fragment therapies, as well as plasmids and enzymes for cutting-edge gene therapy manufacture.

The UK Medicines & Healthcare Products Regulatory Agency on Tuesday published a proposed framework for the recognition of in vitro diagnostics that have already been cleared in other jurisdictions.

A new UK industry body designed to help medical device regulators navigate the new legal landscape of a post-Brexit Britain has launched with backing from 11 companies.

The UK government on Tuesday said it has issued legislation for the implementation of new regulations governing in vitro diagnostics and medical devices.
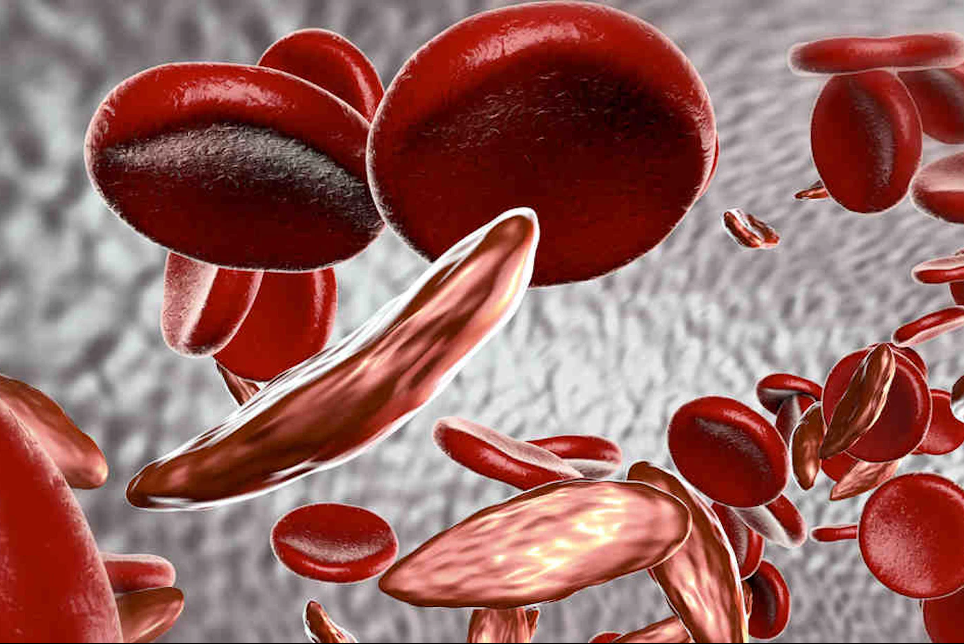
U.K. medical regulators have approved a “world-first” gene therapy that aims to cure two blood disorders: sickle cell disease (SCD) and transfusion-dependent β-thalassemia. They are the first regulatory body to approve the treatment.

MHRA automatically moved the U.K.’s three existing notified bodies, BSI, SGS and UL, to the approved body scheme when the country split from the European Union. Since then, efforts to add capacity have proceeded slowly. The U.K. affiliate of DEKRA, a notified body in the EU, became the first new approved body for medical devices 11 months ago.

Diagnostics company Novacyt UK has agreed to purchase UK-based genomic medicine company Yourgene Health in a £16.7m ($21.3m) deal.
✔ All (7)
✔ Press release (0)
✔ Industry news (7)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
