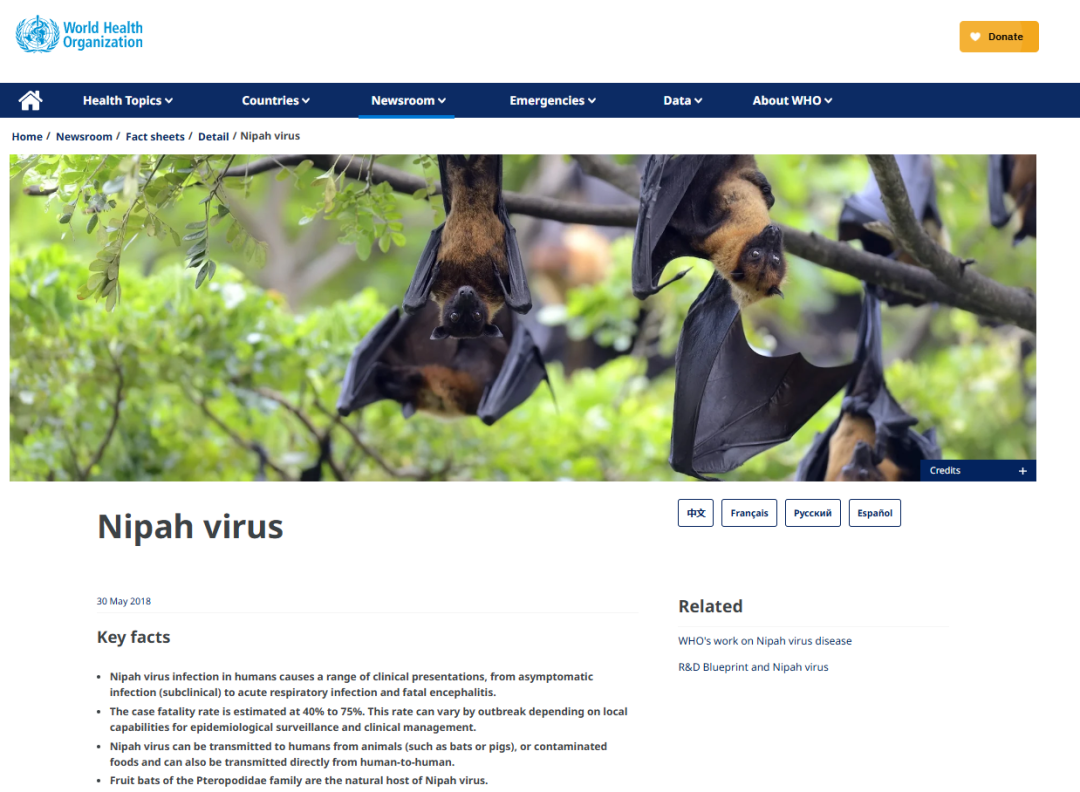
Recently, an outbreak of Nipah virus occurred in West Bengal, India, with 5 confirmed cases reported, including several healthcare workers. Nearly 100 people have been placed under home quarantine, and one patient is in critical condition, highlighting the urgency of epidemic control.

Quanterix Corporation (NASDAQ: QTRX), a company fueling scientific discovery through ultrasensitive biomarker detection, today announced the New York State Department of Health (NYSDOH) has granted the Quanterix Accelerator Laboratory a clinical laboratory permit in the Clinical Chemistry category. The permit approval expands advanced biomarker research led by Quanterix, deepening collaborations with clinical, pharmaceutical, and research partners. The Quanterix Accelerator Lab is now fully CLIA-certified in all 50 states, enabling comprehensive clinical testing and biomarker analysis nationwide.
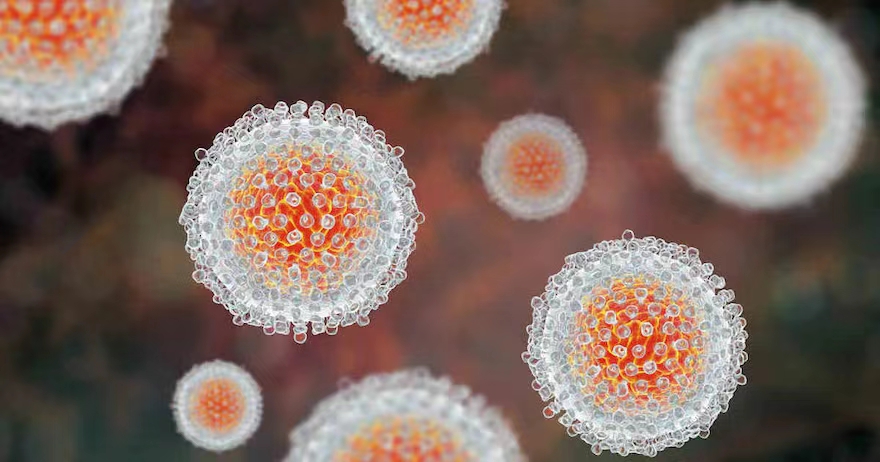
Researchers in Spain have evaluated a minimally invasive test based on dried blood spot samples for hepatitis C RNA detection and genotyping.

The US Food and Drug Administration has allowed InBios International to market its point-of-care anthrax test, the firm announced this week.

Recently, the QuanNUA mass spectrometry system independently developed and manufactured by Intelligene Biosystems (Qingdao) Co., Ltd., the strategic partner of Bioperfectus Technologies, has successfully passed the inspection of Science and Technology Research Center of China Customs.

The independently developed "COVID-19 antigen detection kit SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit (Colloidal gold method)" by Synthgene Medical Technology was approved by the UK 5-year CTDA certification.

On December 26, Hangzhou Biotest Biotech announced that the COVID-19 antigen home self-test test kit produced by Advin, has passed laboratory verification and clinical research evaluation and has now obtained the EUA from the US Food and Drug Administration.
The US Food and Drug Administration said on Thursday it has issued Emergency Use Authorizations to two over-the-counter, at-home antigen tests for detecting the SARS-CoV-2 virus.
The US Food and Drug Administration this week granted Emergency Use Authorization for OnsiteGene's Hi-Sense COVID-19 Molecular Testing Kit 1.0.

Chinese cancer diagnostics firm SeekIn said Monday that it has received the CE mark for its PanCanSeek Cancer Mutation Detection Kit and now intends to market the test in the European Union and other regions that recognize the designation.
✔ All (15)
✔ Press release (1)
✔ Industry news (14)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
