
Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.
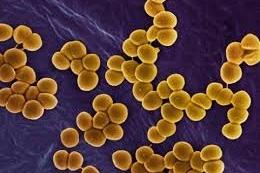
New research suggests that individuals who consistently carry Staphylococcus aureusin their noses have other nasal microbial community features that are distinct from those found in individuals without persistent S. aureus colonization.

Illumina, Inc. (NASDAQ: ILMN) today announced that GeneDx, a leader in genetic testing for rare diseases, is piloting Illumina's emerging constellation mapped read technology, evaluating its performance on regions of the genome that traditional short-read technologies historically have not resolved. GeneDx's early results illustrate the ability of constellation to rapidly identify hard-to-detect variants implicated in rare disease. GeneDx's Director of Laboratory Innovation Joe Devaney will present on the company's early experiences with the constellation technology today at the American Society for Human Genetics (ASHG) Annual Meeting in Boston.

Private equity group Warburg Pincus is nearing a deal to take a large minority stake in French diagnostics group Sebia, which values the business at €5.4bn, according to people familiar with the matter.

Myriad Genetics (Nasdaq: MYGN), a leader in molecular diagnostic testing and precision medicine, and SOPHiA GENETICS (Nasdaq: SOPH), an AI technology company transforming precision medicine, announced a strategic collaboration to develop and provide pharmaceutical companies with an innovative global liquid biopsy companion diagnostic (CDx) test. This partnership will leverage Myriad’s advanced laboratory capabilities in the U.S. to support global testing for clinical trials and SOPHiA GENETICS’ broad, decentralized network of more than 800 connected institutions in more than 70 countries for global test deployment.

RetinalGenix Technologies Inc. OTCQB:RTGN (“RetinalGenix” or the “Company”), a pioneering developmental-stage company focused on ophthalmic screening, monitoring, pharmacogenetic mapping, and repurposed drug development for early detection and treatment of eye and systemic diseases, has entered into an agreement with LabCorp, one of the nation’s largest laboratory services organizations, to support the rollout of the RetinalGenix DNA/RNA/GPS Pharmaco-Genetic Mapping™ platform. This innovative program enables patients to undergo genetic testing and high-resolution retinal imaging anonymously and provide insights into both ocular and systemic diseases.

Eli Lilly and Company (NYSE: LLY) and Verve Therapeutics, Inc. (Nasdaq: VERV), a Boston-based clinical-stage company developing genetic medicines for cardiovascular disease, today announced a definitive agreement for Lilly to acquire Verve.

QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) today announced it has signed a definitive agreement to acquire Genoox, a provider of AI-powered software that enables clinical labs to scale and accelerate the processing of complex genetic tests.

Dutch diagnostics firm MRC Holland said Thursday that its liquid biopsy test for Peutz-Jeghers syndrome has been certified under Europe's In Vitro Diagnostic Regulation (IVDR).

In vitro fertilization embryos are prone to chromosomal aneuploidy abnormalities. Chromosome aneuploidy refers to the increase or decrease in the number of a single chromosome or multiple chromosomes.
✔ All (31)
✔ Press release (0)
✔ Industry news (31)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
