
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that it has received CE mark for its Elecsys® Dengue Ag test – a high-throughput, fully automated immunoassay to be used as an aid in the diagnosis of an acute dengue virus infection. This milestone promises to set a new standard for efficiency and reliability in tackling the growing global challenge of dengue fever.
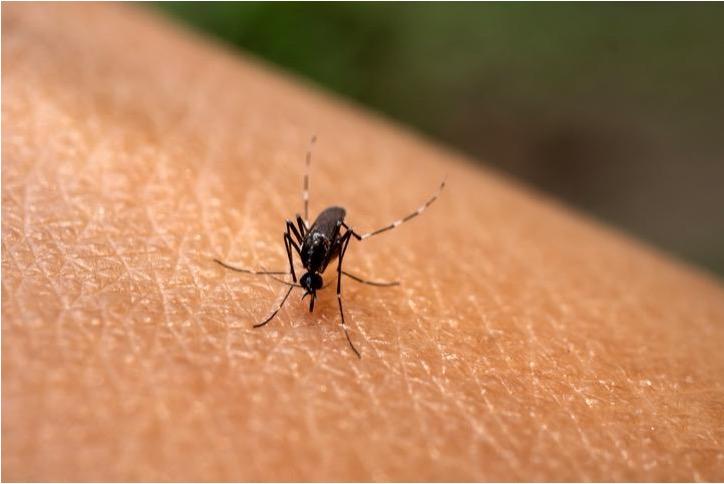
Rapid diagnostic tests for dengue fever will be accessible at all airports across the country starting Friday, the Korea Disease Control and Prevention Agency (KDCA) said, Thursday. This comes in response to the rising number of domestic cases, where individuals have been contracting the virus through mosquito bites during their overseas travels.
✔ All (2)
✔ Press release (0)
✔ Industry news (2)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
