
Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced that the U.S. Food and Drug Administration (FDA) has approved Guardant360® CDx as a companion diagnostic to identify patients with BRAF V600E-mutant metastatic colorectal cancer (mCRC) who may benefit from treatment with BRAFTOVI® (encorafenib) in combination with cetuximab and chemotherapy in accordance with the approved product labeling.

Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced a multi-year collaboration with Merck, known as MSD outside the United States and Canada, to support the development and commercialization of Merck’s oncology portfolio using the Guardant Infinity™ Smart platform.

The US Food and Drug Administration on Tuesday released a proposal to reclassify companion diagnostic assays from Class III medical devices to Class II devices.

Thermo Fisher Scientific, the world leader in serving science, has received approval from the U.S. Food and Drug Administration (FDA) for its Oncomine Dx Target Test as a companion diagnostic (CDx) to identify patients who may be candidates for HERNEXEOS® (zongertinib tablets), a tyrosine kinase inhibitor (TKI), developed by Boehringer Ingelheim. The test allows clinicians and pathologists to assess if non-small cell lung cancer (NSCLC) tumors harbor human epidermal growth factor receptor 2 (HER2/ERBB2) tyrosine kinase domain (TKD) activating mutations.

The U.S. Food and Drug Administration (FDA) has approved the Promega OncoMate® MSI Dx Analysis System as a companion diagnostic designed to identify patients with microsatellite stable (MSS; defined as not MSI-high [not MSI-H]) endometrial carcinoma who may benefit from treatment with KEYTRUDA® (pembrolizumab), Merck’s anti-PD-1 therapy, plus LENVIMA® (lenvatinib), the orally available multiple receptor tyrosine kinase inhibitor discovered by Eisai. This is the first Promega companion diagnostic to receive FDA approval.

Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced that the U.S. Food and Drug Administration (FDA) has approved its Guardant360® CDx as a companion diagnostic to identify advanced breast cancer patients with ESR1 mutations who may benefit from Eli Lilly and Company’s Inluriyo (imlunestrant). Guardant360 CDx was approved in conjunction with Inluriyo for the treatment of adults with estrogen receptor-positive (ER+), human epidermal growth factor receptor 2-negative (HER2–), ESR1-mutated advanced or metastatic breast cancer whose disease progressed after at least one line of endocrine therapy (ET).

Myriad Genetics (Nasdaq: MYGN), a leader in molecular diagnostic testing and precision medicine, and SOPHiA GENETICS (Nasdaq: SOPH), an AI technology company transforming precision medicine, announced a strategic collaboration to develop and provide pharmaceutical companies with an innovative global liquid biopsy companion diagnostic (CDx) test. This partnership will leverage Myriad’s advanced laboratory capabilities in the U.S. to support global testing for clinical trials and SOPHiA GENETICS’ broad, decentralized network of more than 800 connected institutions in more than 70 countries for global test deployment.
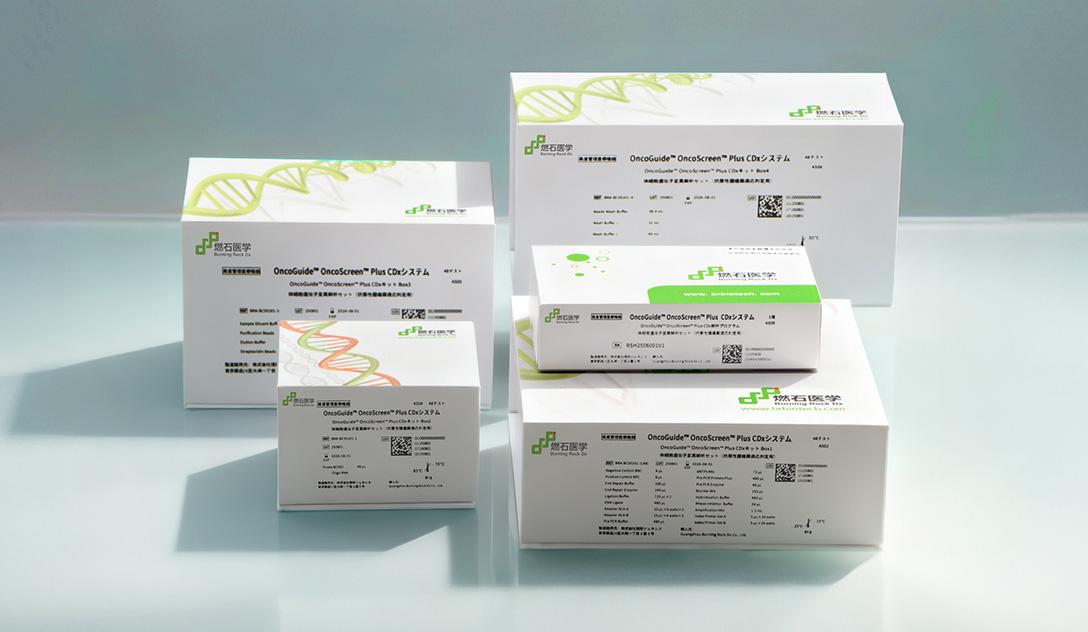
Riken Genesis Co., Ltd. (Riken Genesis) and Burning Rock Biotech Limited (NASDAQ: BNR, “Burning Rock”) today announced that the OncoGuide™ OncoScreen™ Plus CDx System based on OncoScreen™ Plus to be used as a companion diagnostic for AstraZeneca’s capivasertib has received Manufacturing and Marketing Approval from Japan’s Ministry of Health, Labour and Welfare (MHLW).

Lunit (KRX:328130.KQ), a leading provider of AI for cancer diagnostics and therapeutics, and Agilent Technologies Inc. (NYSE: A), a global leader in life sciences, diagnostics, and applied chemical markets, today announced a nonexclusive collaboration to develop AI-based companion diagnostic solutions. The collaboration will leverage Lunit's AI technology and Agilent's expertise in tissue-based companion diagnostics to create advanced solutions that meet the demand of novel and complex biomarker assays in drug development.

Biocartis Group of Companies (“Biocartis”), an innovative molecular diagnostics company, is pleased to announce that its Idylla™ CDx MSI Test developed in partnership with Bristol Myers Squibb, has received the first-ever Premarket Approval (PMA) from the FDA for a cartridge-based, fully automated, “sample-to-result” companion diagnostic test.
✔ All (88)
✔ Press release (0)
✔ Industry news (88)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
