
Adaptive Biotechnologies said last week that its new subsidiary Digital Biotechnologies has held an initial closing of its Series A preferred stock financing and expects to raise up to $15 million in the round.

Guardant Health Japan said Monday that it has received approval from that country's Ministry of Health, Labour, and Welfare (MHLW) for its Guardant360 CDx as a companion diagnostic for identifying ESR1 mutations in breast cancer patients.

Results of a Phase I clinical trial published on Tuesday demonstrate that Personalis' minimal residual disease (MRD) assay, NeXT Personal, accurately predicts immunotherapy response across multiple metastatic solid tumor cancer types.

Molecular diagnostics firm BillionToOne reported after the close of the market on Tuesday that its Q3 2025 revenues more than doubled year over year.

Swedish diagnostics firm Devyser said Thursday that its next-generation sequencing-based test for stem cell transplantation monitoring has received regulatory approval in Canada.

As part of its strategic expansion into precision dermatology, Castle Biosciences recently introduced a new assay to guide systemic treatment decision making for the immune-driven disease atopic dermatitis. Castle expects to expand the limited launch of its AdvanceAD-Tx test over the next year to pursue a total addressable market that it estimates to be as much as $33 billion.
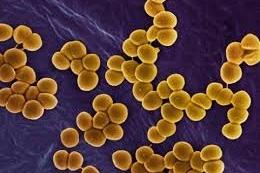
New research suggests that individuals who consistently carry Staphylococcus aureusin their noses have other nasal microbial community features that are distinct from those found in individuals without persistent S. aureus colonization.

JP Morgan, BTIG, and Jefferies announced separately Monday that they have initiated coverage of molecular diagnostics firm BillionToOne.

Qiagen is partnering with Oxford Gene Technology, a Sysmex subsidiary, to comarket and distribute its Qiagen Clinical Insight (QCI) Interpret software with OGT's SureSeq NGS panels, the firms announced Thursday. Financial terms were not disclosed.

MGI Tech has reported that its revenues for the first half of 2025 dropped 8 percent year over year, mainly due to declining instrument sales.
✔ All (54)
✔ Press release (0)
✔ Industry news (54)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
