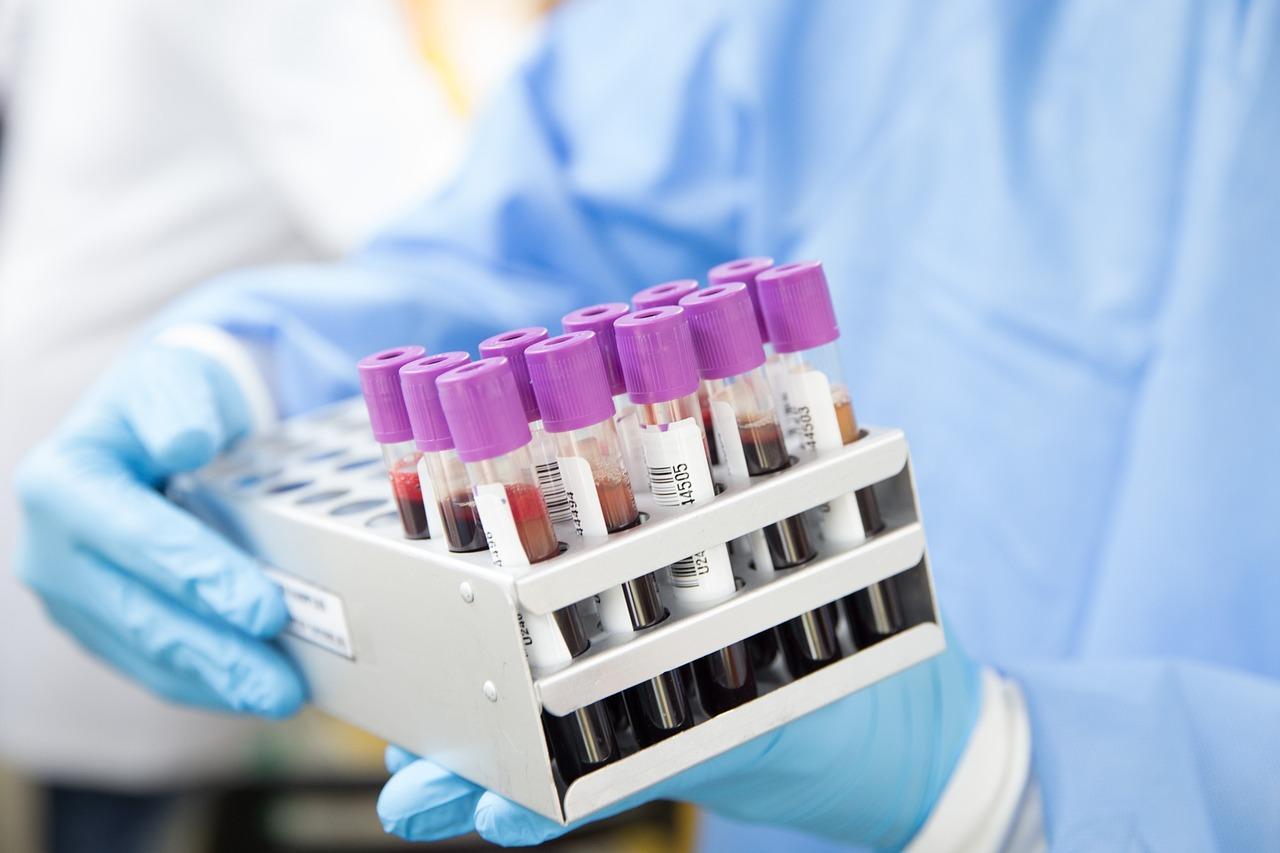
Automatic blood grouping analyzer is an analyzer that can forward and reverse ABO blood group, Rh (D) blood group test, irregular antibody screening and cross-matching of blood test. It mainly consists of liquid handling system, incubator, centrifuge, interpretoscope, and microcomputer system.

Guardant Health has acquired MetaSight Diagnostics for $59 million in upfront cash to bolster its multi-disease detection pipeline, the company said Thursday. The deal includes up to $90 million in payments tied to future commercial performance and regulatory approvals.
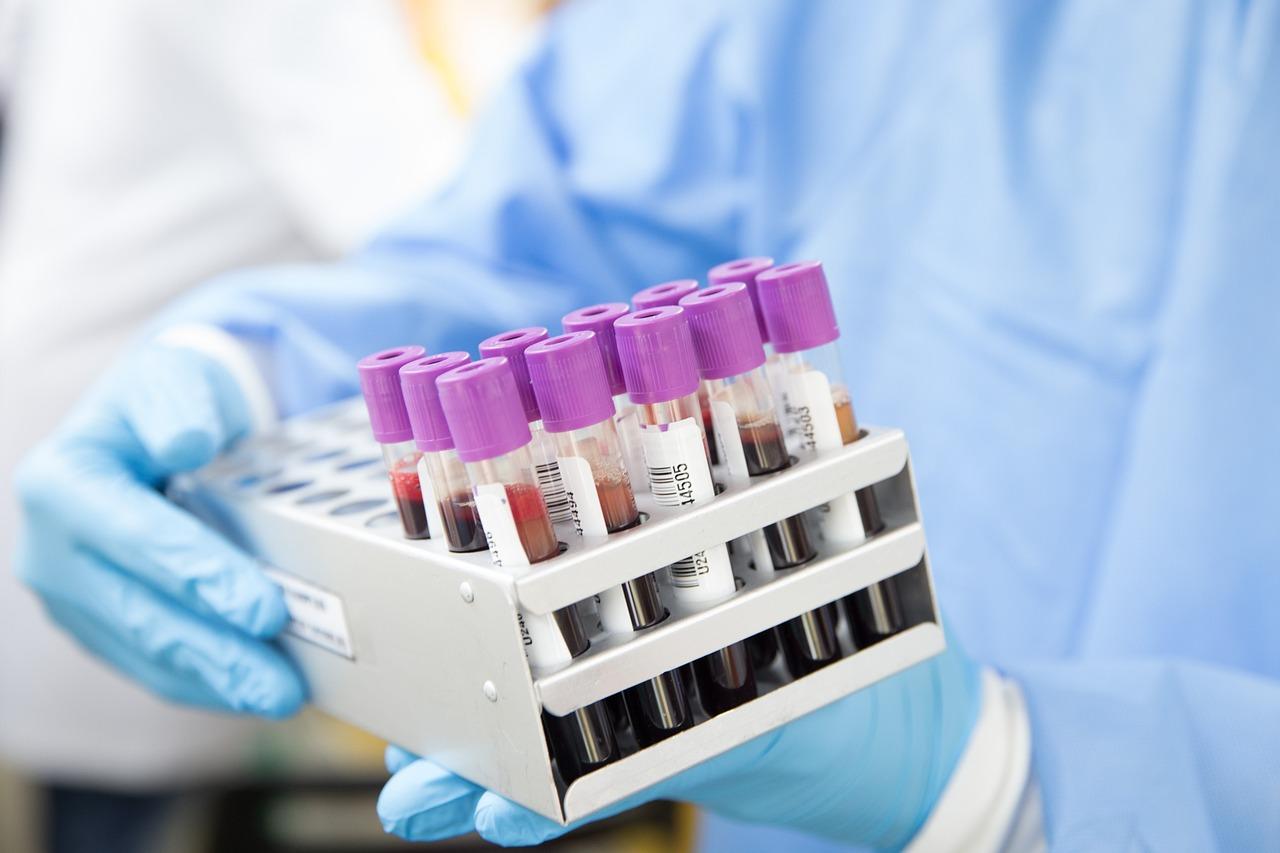
Blood typing-related products mainly include two sectors of blood typing instruments and blood typing reagents.

Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.

Truvian Health (“Truvian”), a diagnostics company redefining routine blood testing, today announced U.S. Food and Drug Administration (FDA) clearance for the Complete Blood Count (CBC) on its TruVerus™ multi‑modal blood testing system (K251249).

According to the results of the basic research, different medical institutions have selected different imported and domestic brand instruments: 38 domestic and 58 imported.

Coagulation analyzers are mainly applied for laboratory tests of thrombosis and hemostasis, which can provide valuable indicators for the diagnosis and differential diagnosis of hemorrhagic and thrombotic diseases, detection, and efficacy of thrombolytic and anticoagulant therapy.

Cardiovascular testing firm Prevencio and Quadrant Health said Thursday that they have entered an agreement to integrate Prevencio's HART CVE and HART CADhs blood tests into Quadrant's primary care workflows to better translate the test results into clinical action and measure outcomes.

As indicated by the release of the China Cardiovascular Health and Disease Report 2024, the current number of cardiovascular patients is 330 million and is still on the rise.

Against the background of an increasingly aging global population, the incidence of thrombotic and hemostatic diseases, including cardiovascular diseases, remains high.
✔ All (88)
✔ Press release (0)
✔ Industry news (88)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
