


Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

The research describes a new and fast computational approach to obtain precisely dated evolutionary trees, known as ‘timetrees’.

Novo Nordisk today announced that the acquisition of Dicerna Pharmaceuticals, Inc. (Dicerna; Nasdaq: DRNA), announced on 18 November 2021, has been completed.

Laboratory Corporation of America said Thursday that it has agreed to acquire cancer genomics firm Personal Genome Diagnostics (PGDx) for $450 million in cash and $125 million in additional payments based on future performance milestones.

On December 28,Shanghai Universal Biotech Co.,Ltd. was listed on the Shenzhen Stock Exchange (SZSE) ChiNext, stock code: 301166. The issue price is 86.06 yuan/share, and the opening price is 92 yuan/share.
Scientists at Scripps Research, University of Chicago and Icahn School of Medicine at Mount Sinai have identified a new Achilles’ heel of influenza virus, making progress in the quest for a universal flu vaccine.

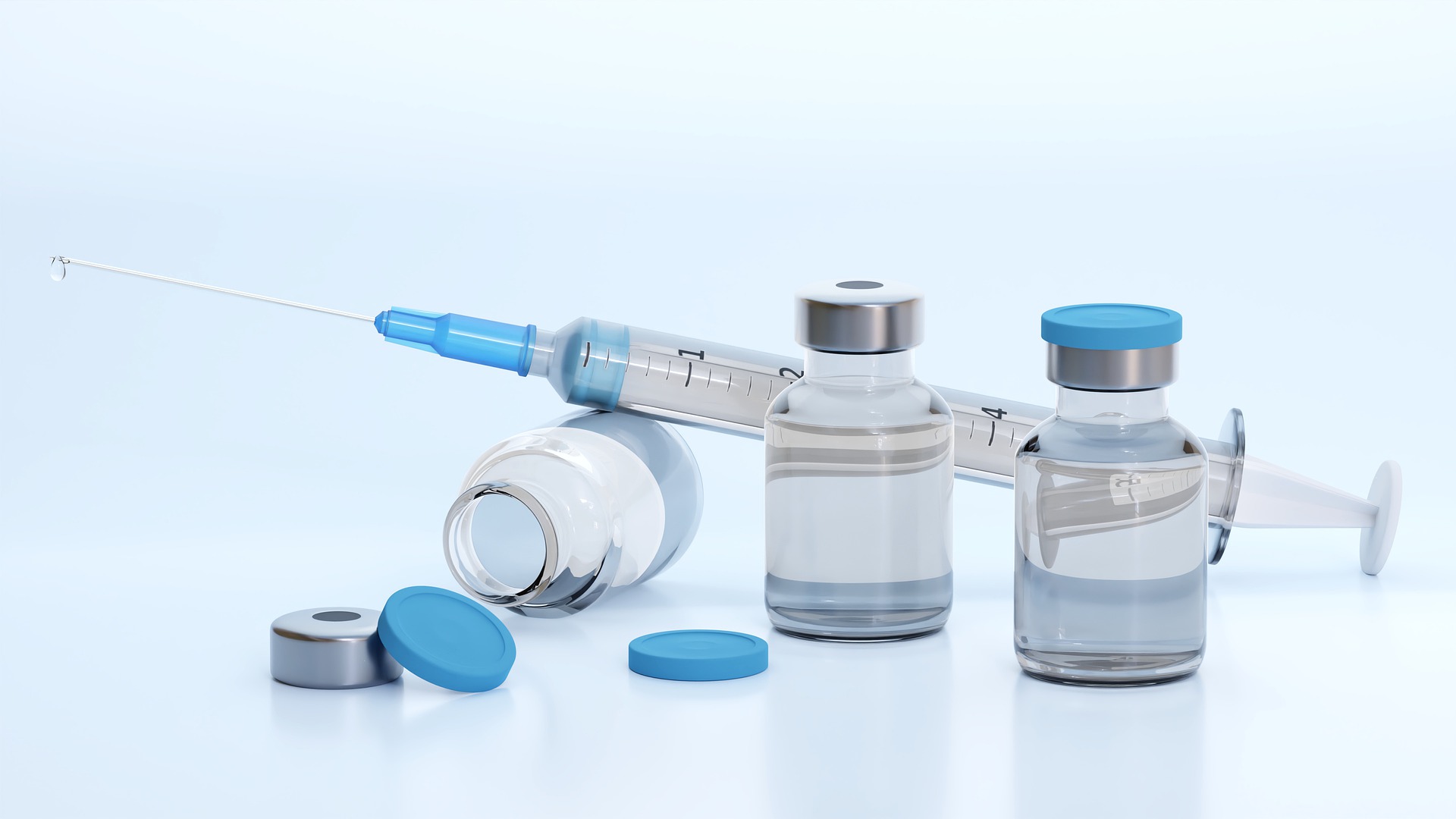
Scientists have identified unique “indicators” in the blood of patients with severe and fatal COVID, paving the way for simple diagnostic tests to help doctors identify who will go on to become critically ill.

After you finish a milkshake or a bowl of guacamole, those dietary fats have a typical route through your body. New research from the laboratory of Dr. Natasza Kurpios, has now revealed a key molecular mechanism.

LumiraDx said on Thursday that it has received CE marking for its point-of-care SARS-CoV-2 & Flu A/B Antigen Test.

Quidel Corporation (NASDAQ: QDEL) (“Quidel”) and Ortho Clinical Diagnostics Holdings plc (NASDAQ: OCDX) (“Ortho”) today announced that they have entered into a definitive agreement in which Quidel will acquire Ortho

On December 15th, Ministry of Science and Technology of the People’s Republic of China announce the list of advanced collectives in the national science and technology system for fighting COVID-19 epidemic.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
