

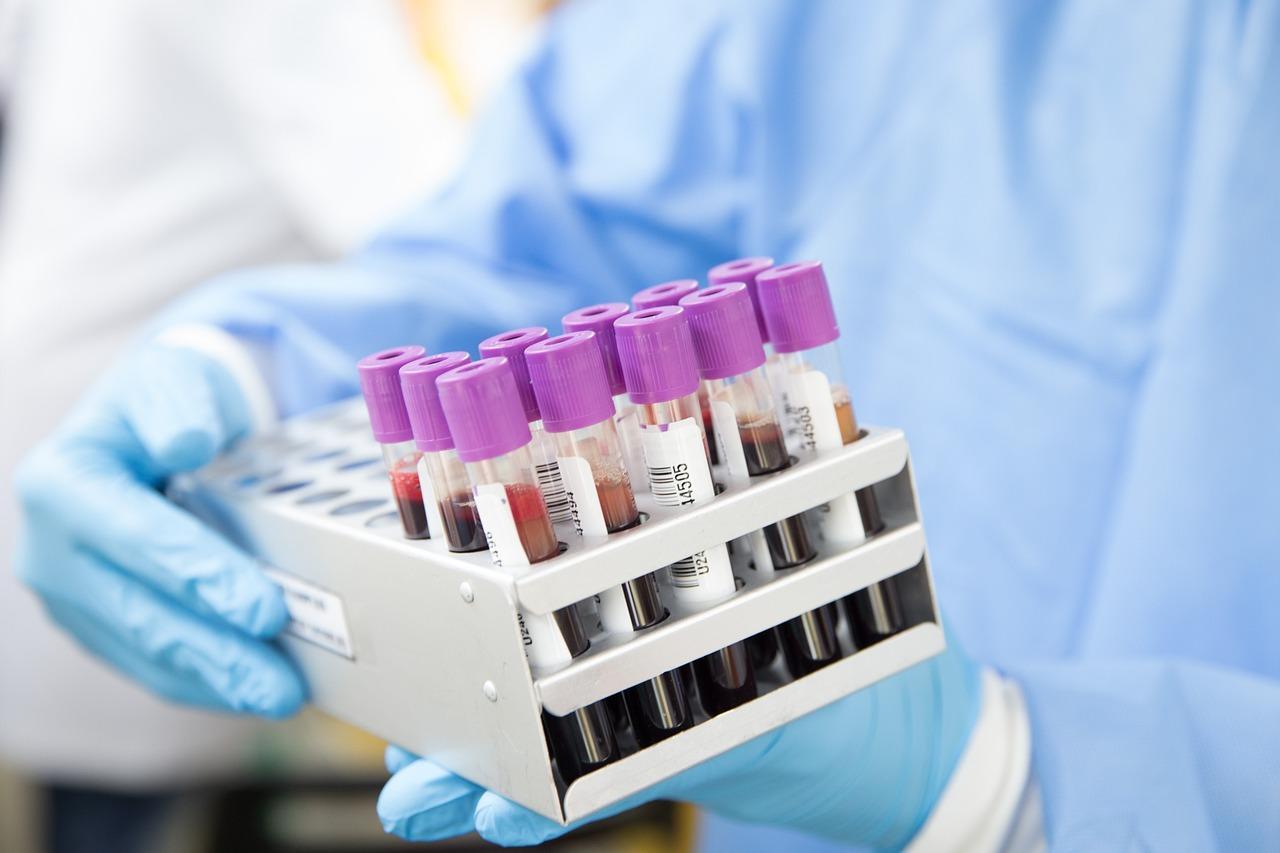
Blood typing reagents mainly refer to reagents and kits used separately or in combination with blood grouping analyzer, which belong to a segment of in vitro diagnostic (IVD) reagent industry.
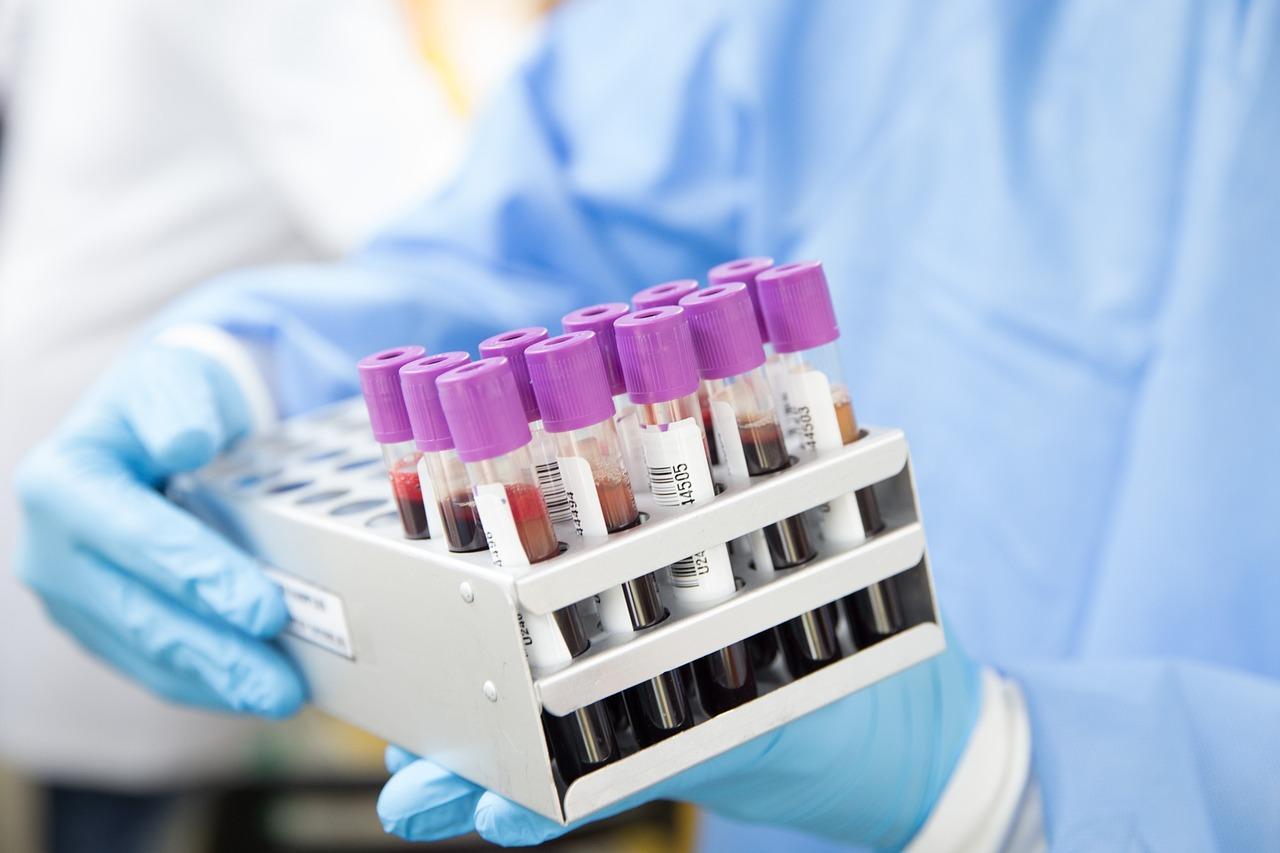
Automatic blood grouping analyzer is an analyzer that can forward and reverse ABO blood group, Rh (D) blood group test, irregular antibody screening and cross-matching of blood test. It mainly consists of liquid handling system, incubator, centrifuge, interpretoscope, and microcomputer system.

Horizon Therapeutics has partnered with Invitae for its urea cycle disorder genetic (UCD) testing program, through which individuals who may have the rare genetic condition and their family members will receive testing at no charge.

Quanterix announced after the close of the market on Wednesday that it has inked an agreement to acquire privately held UmanDiagnostics for $22.5 million.

Illumina has filed patent infringement suits in the US, Switzerland, and Turkey relating to BGI's sequencing instrumentation and chemistry, the company said today.

The global point of care (POC) diagnostics/testing market size is expected to grow at a CAGR of 3.3% over the forecast period 2019-2025 to reach USD 22.8 billion by 2025, driven by the ability of POC tests to render rapid and accurate resu

The US Food and Drug Administration has approved Qiagen's Therascreen PIK3CA RGQ PCR Kit as a companion diagnostic for identifying which advanced breast cancer patients have PIK3CA mutations and are likely to respond to Novartis' alpelisib (

Roche said recently that it has received US Food and Drug Administration 510(k) clearance for its Cobas TV/MG test for the detection of Trichomonas vaginalis (TV) and Mycoplasma genitalium (MG) DNA in symptomatic and asymptomatic patients.

PreludeDx said recently that the State of New York Clinical Laboratory Evaluation Program (CLEP) has approved its DCISionRT test, which assesses recurrence risk and can predict radiation therapy benefit in patients diagnosed with non-invasiv

Turning Point Therapeutics and Almac Diagnostic Services recently announced approval by the US Food and Drug Administration of an investigational device exemption for use of an Almac diagnostic assay to determine whether cancer patients are

German molecular diagnostics firm Curetis said recently that it will receive €6.5 million ($7.3 million) in gross proceeds under two financing facilities.
NeoGenomics said after the close of the market recently that it has priced its previously announced underwritten public offering of 7 million shares of its common stock at a public offering price of $21.25 per share.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
