Original from: yaoZH
1. Overall
1.1 Trend of domestic medical device product registration & filing, 2019-2023
Up to now, the total number of domestic Class II & III medical device product registrations within the validity period in China has reached 114,626.
From 2019 to 2022, the number of registrations rises year by year. The total number of product registrations is 23,127 as of now in 2023, with a growth rate of 17.7% in 2022. During the same period, Class I device filings also show an upward trend, with a growth rate of 11.0% in 2022; the total number of Class I device filings is 33,015 as of 2023.


1.2 Medical device registration & filing in Chongqing
There are 2,473 registered medical devices and 1,651 approved and filed medical devices in Chongqing.
1.3 Trend of Chongqing medical device product registration & filing, 2019-2023
The total number of medical device product registrations in Chongqing within the validity period has reached 2,473. The number of registrations shows a rising trend year by year from 2019 to 2023. The total number of product registrations is 1,139 as of now in 2023, with a growth rate of up to 89.4% in 2022.
During the same period, the number of filings also shows an upward trend, with the total number of product filings standing at 360 as of 2023; the growth rate reaches 26.3% in 2022.


2. Detailed Analysis
2.1 Analysis of the number of source places of domestic class II medical device registered product sources in 2023
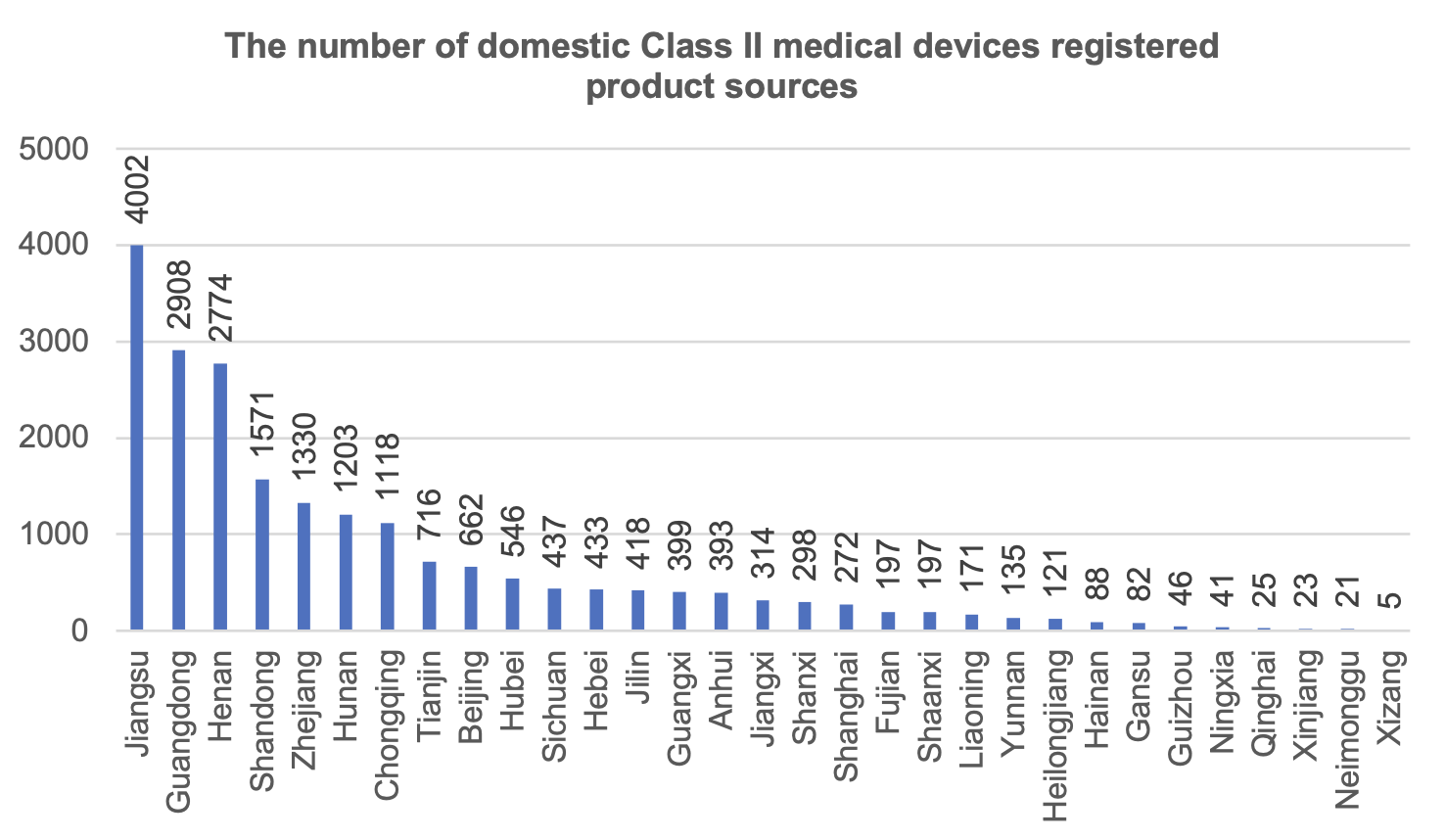
From the distribution of the source of Class II medical device registered products, 31 provinces and cities (excluding Hong Kong, Macao and Taiwan) registered Class II medical devices in 2023, of which Jiangsu Province registered the largest number of medical devices, accounting for 19.1% of the registered number of domestically produced Class II medical devices.
Chongqing ranked 7th in the number of Class II medical device product registrations, with the number of registrations accounting for 5.3% of the country, ahead of Sichuan Province.

2.2 Analysis of the number of source places of domestic class III medical device registered product sources in 2023
According to the distribution of the source places of Class III medical device registered products, a total of 27 provinces and municipalities (excluding Hong Kong, Macao and Taiwan) registered Class III medical devices in China in 2023.
Among them, Jiangsu Province ranked first in the number of registered medical devices, accounting for 20.4% of the total number of domestic Class III medical device registrations in China. Chongqing ranked 17th in the number of Class III medical device product registrations, accounting for 0.9% of the national total, relatively behind Sichuan Province.


2.3 Chongqing medical device product registration
From the perspective of product registration, the number of medical device products registered for the first time in Chongqing is 959, the number of extended registrations is 1,356.


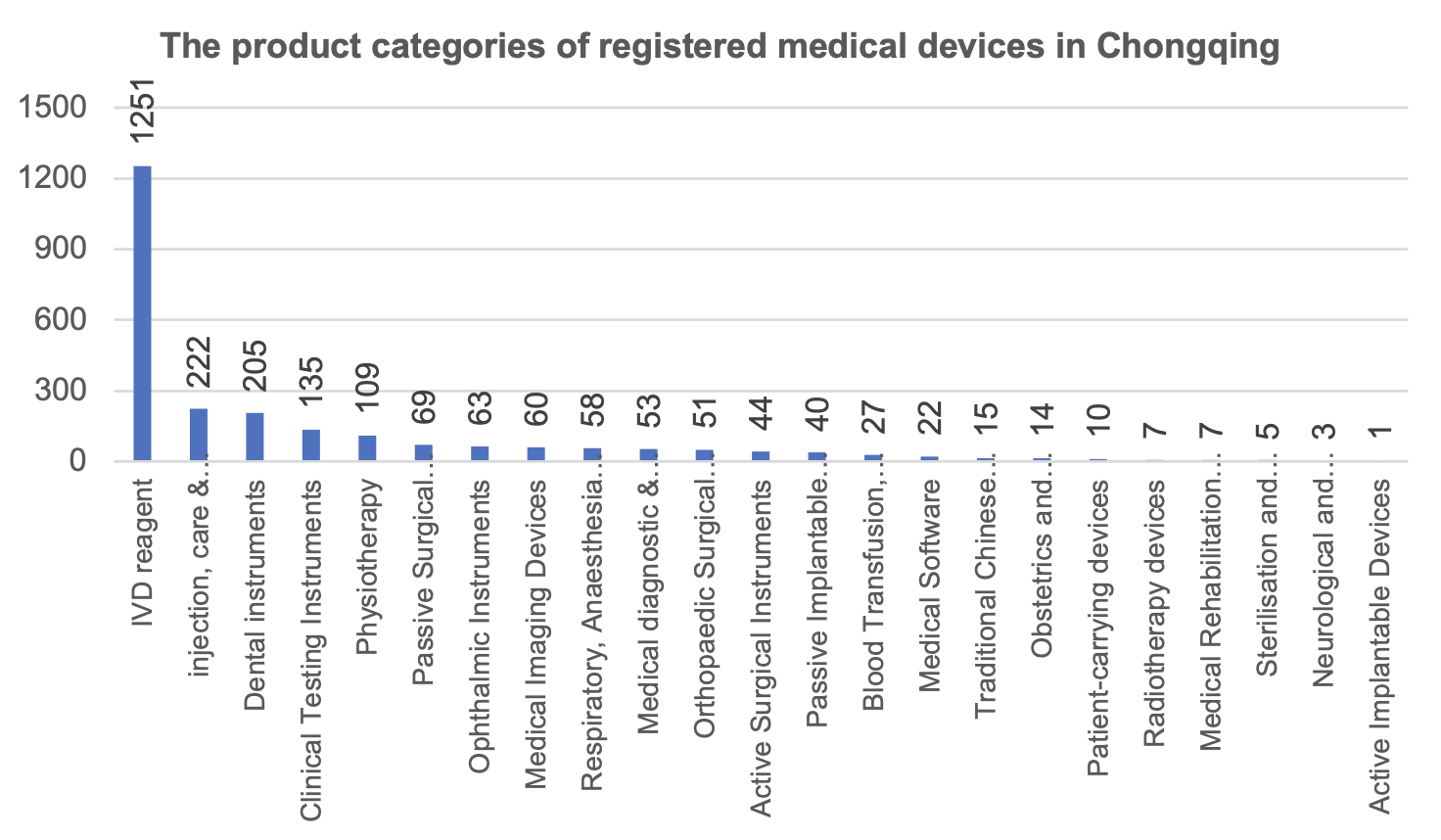
From the perspective of product categories, in vitro diagnostic reagents (1,251 pieces), injection, care and protection devices (222 pieces) and stomatological devices (205 pieces) are the top three registered medical devices in Chongqing.

2.4 Chongqing companies’ registered class II medical device products (top10)
From the number of companies that registered for medical devices, as of November 2023, there are 343 companies registered class II medical device products in Chongqing within the validity period, and the top one is Zybio Inc. with 302 product registrations.
Among them, a total of 175 companies registered class II medical device products in Chongqing in 2023, and the top one in the number of registrations is Zybio Inc. with 226 product registrations.
2.5 Chongqing companies’ registered class III medical device products (top10)
As of November 2023, there are 46 companies registered class III medical device products in Chongqing within the validity period, and the top one is Forwos Medical with 23 product registrations.
Among them, a total of 10 companies registered class III medical device products in Chongqing in 2023, and the top one in the number of registrations is Chongqing Xike Medical Technology Co., Ltd. with 4 product registrations.
3. Breakthrough Medical Devices
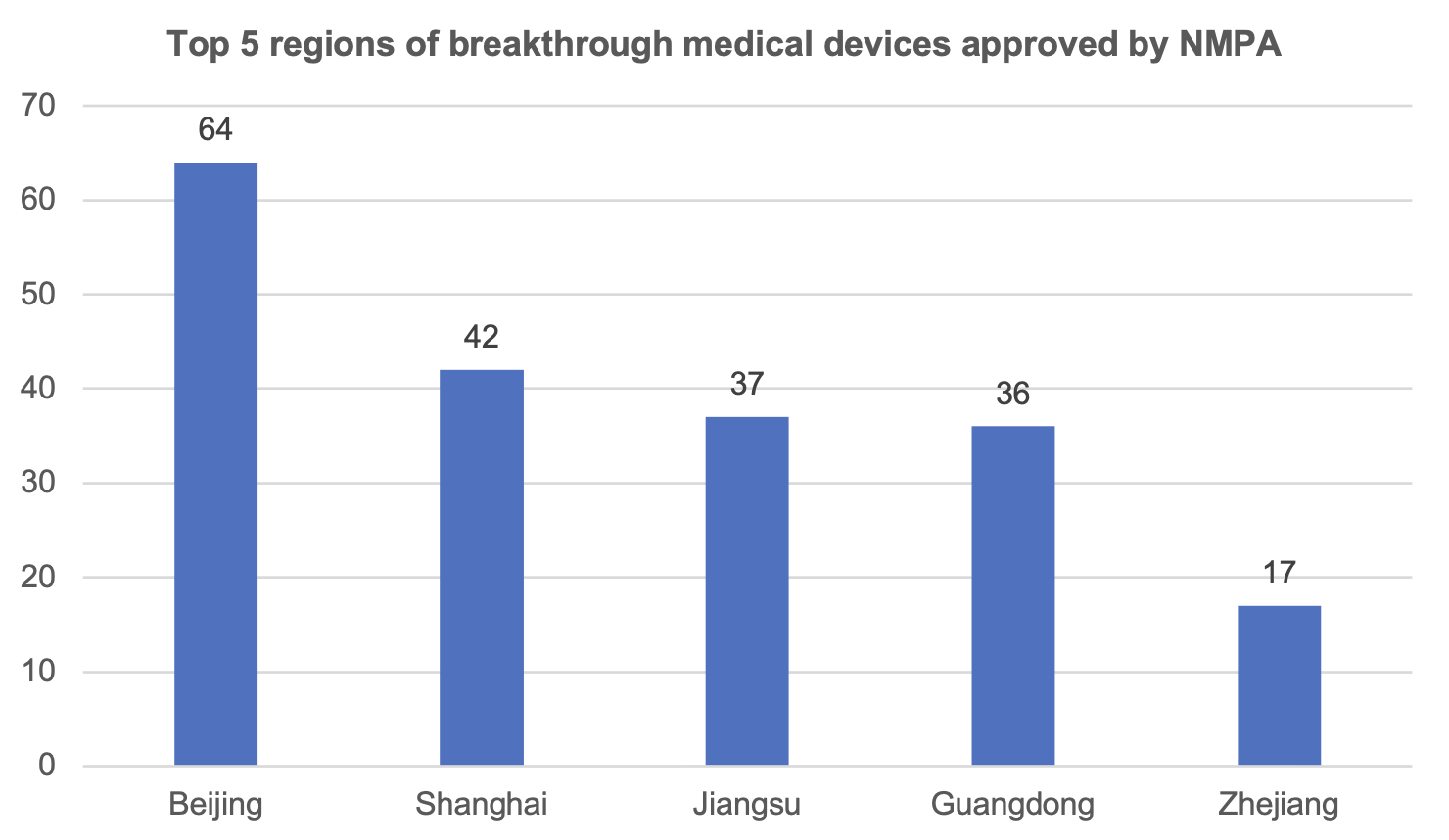
According to statistics, the National Medical Products Administration has approved 245 breakthrough medical devices to be listed on the market.
Among them, Beijing has 64 products approved to be listed, ranking the first in the country; Shanghai has 42 products approved to be listed, ranking the second in the country. Chongqing has 2 breakthrough medical device products on the market.


Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
