


Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

The Global Health Innovative Technology (GHIT) Fund said Monday that it has invested ¥70 million ($460,000) in the development of a prototype molecular assay to detect mpox.

Against the background of an increasingly aging global population, the incidence of thrombotic and hemostatic diseases, including cardiovascular diseases, remains high.

Paradigm Health announced last Thursday that it acquired Flatiron Health's clinical research business, establishing a multiyear collaboration between the two companies.

Natera announced last Friday that it has acquired cancer diagnostic firm Foresight Diagnostics for $275 million upfront.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Swedish diagnostics firm Devyser said Thursday that its next-generation sequencing-based test for stem cell transplantation monitoring has received regulatory approval in Canada.

Agilent Technologies Inc. (NYSE: A) held a ribbon-cutting ceremony at the Shanghai Waigaoqiao New Development Park to celebrate the 30th anniversary of the Agilent Shanghai Manufacturing Center (ATS) and announce the official opening of its new Chromatography Column Manufacturing Center.
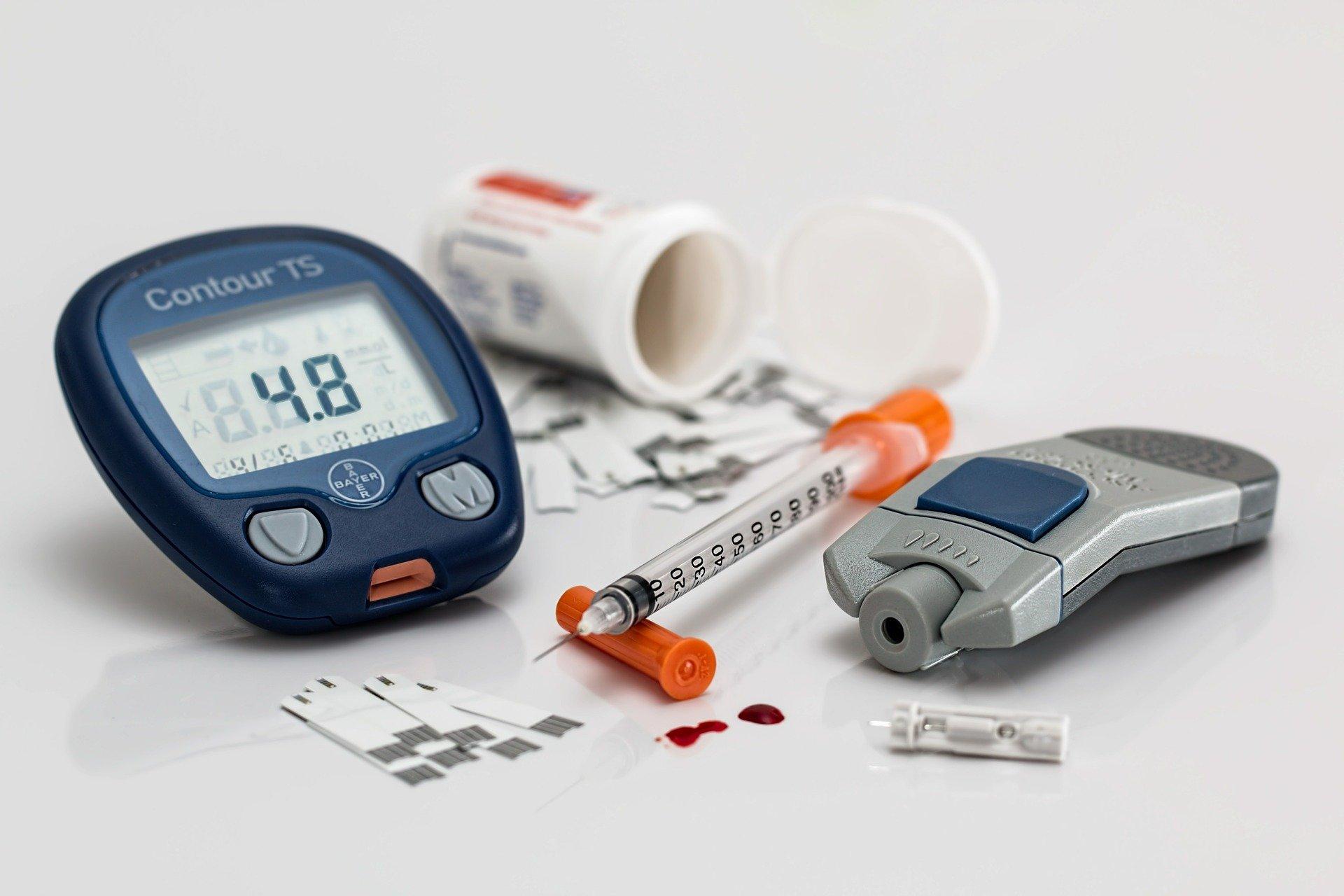
Scout Health announced Wednesday that it has been awarded up to $6 million in funding support from the antimicrobial resistance nonprofit CARB-X. The firm plans to use the funding to finalize development of a next-generation version of its rapid point-of-care system and assay for chlamydia and gonorrhea and hopes to soon begin pilot studies in low- and middle-income countries (LMICs).

As part of its strategic expansion into precision dermatology, Castle Biosciences recently introduced a new assay to guide systemic treatment decision making for the immune-driven disease atopic dermatitis. Castle expects to expand the limited launch of its AdvanceAD-Tx test over the next year to pursue a total addressable market that it estimates to be as much as $33 billion.

On December 2, 2025, Merck announced that Rogier Janssens will officially assume the role of President of Merck China starting February 1, 2026.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
