


Different medical institutions have selected different imported and domestic brand instruments: 38 domestic and 58 imported.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

PerkinElmer, Inc. (NYSE: PKI) (“PerkinElmer”) today announced it has entered into an agreement to acquire BioLegend, a leading, global provider of life science antibodies and reagents, for approximately $5.25 billion in a combination of cash and stock, subject to certain adjustments.

On July 25, 2021, Autobio and Syscan officially announced the launch of a strategic cooperation. In the future, the two companies will make full use of their respective technology and channel advantages to carry out a full range of in-depth cooperation in the field of coagulation testing.

July 22, Roche reported a 51 percent increase in half-year revenues for its Diagnostics division, largely thanks to demand for its COVID-19 products.

Danaher reported before the opening of the market on Thursday that its second quarter revenues rose 37 percent year over year, thanks largely to steep gains from its life sciences and diagnostics businesses.

July 22, Abbott said that its Diagnostics business revenues grew 63 percent year over year in the second quarter, while its total revenues were up 39 percent.
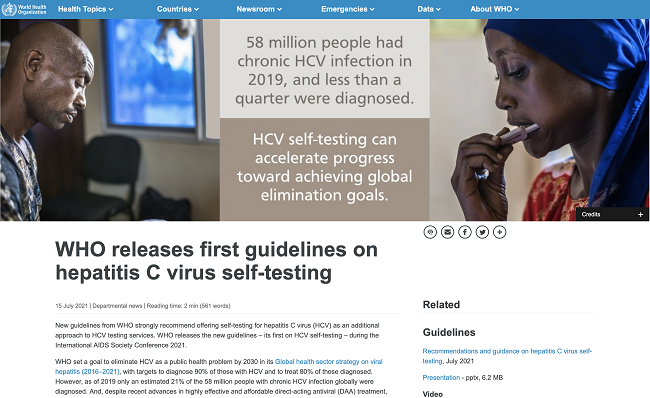
New guidelines from WHO strongly recommend offering self-testing for hepatitis C virus (HCV) as an additional approach to HCV testing services. WHO releases the new guidelines – its first on HCV self-testing – during the International AIDS Society Conference 2021. 
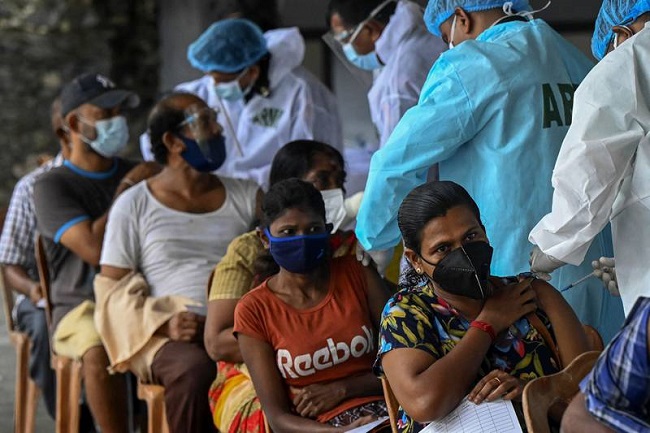
Research in Sri Lanka shows more than 80 per cent of those inoculated develop antibodies capable of neutralising the virus
Agilent Technologies on Tuesday announced that it has received CE marking for the expanded use of its PD-L1 IHC 22C3 pharmDx companion diagnostic assay in patients with non-small cell lung cancer.

Runda Medical issued a performance forecast, and it is expected that the net profit attributable to shareholders of listed companies will be between 195 million yuan and 215 million yuan in the first half of 2021, an increase of 75.33% year-on-year to 93.31%.
Recently, Fosun Pharma announced that the approval of the mRNA novel coronal vaccine by the Food and Drug Administration has been basically completed, and the expert review has passed. The administrative approval stage is being stepped up at present.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
