


Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Abbott (NYSE: ABT) today announced U.S. Food and Drug Administration (FDA) clearance for two new over-the-counter continuous glucose monitoring (CGM) systems – Lingo™ and Libre Rio™, which are based on Abbott's world-leading FreeStyle Libre® continuous glucose monitoring technology1, now used by about 6 million people globally2. The newly cleared systems have been intentionally designed to meet different needs – Lingo for consumers who want to better understand and improve their health and wellness, and Libre Rio for adults with Type 2 diabetes who do not use insulin and typically manage their diabetes through lifestyle modifications.

Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that the U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for its cobas® liat SARS-CoV-2, Influenza A/B & RSV nucleic acid test, an automated multiplex real-time polymerase chain reaction (RT-PCR) assay on the cobas® liat system. Producing results in just 20 minutes on a compact analyser suitable for most healthcare settings, the test uses either a single nasopharyngeal or anterior nasal-swab sample to confirm or rule out infection with SARS-CoV-2, influenza A virus, influenza B virus and respiratory syncytial virus (RSV).
The Association for Molecular Pathology on Thursday published a new set of evidence-based recommendations for the analytical validation and reporting of tumor mutational burden (TMB) testing.

DELFI Diagnostics, Inc., a developer of accessible blood-based tests that deliver a new way to enhance cancer detection, today announced an equity investment by the Merck Global Health Innovation Fund (GHIF), the corporate venture capital arm of Merck & Co., Inc, known as MSD outside the United States and Canada. The capital will accelerate and expand DELFI's development and commercialization of its cancer detection solutions. DELFI's fragmentomics technology applies artificial intelligence (AI) to whole-genome sequencing data to compare an individual's cell-free DNA (cfDNA) patterns and characteristics against populations with and without cancer.

Diasorin today announces that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the company’s new LIAISON PLEX® Yeast Blood Culture (BCY) Assay, the second molecular multiplexing panel on the LIAISON PLEX® system.

QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) today announced the launch of its new digital PCR (dPCR) Custom Assay Design Tool for copy number variation (CNV) analysis for use on its digital PCR platform QIAcuity and several other enhancements in its GeneGlobe Design and Analysis Hub, a comprehensive research platform that integrates pre-designed assays with a database of more than 10,000 biological entities including genes, miRNAs, and pathways. The new advancements aim to support customers with a wide range of assay customization options, from simple to complex and validated multiplex assays, while further improving the user experience.

ELITechGroup is pleased to announce that they have obtained the IVDR certification for four new products including the brand new CMV RNA ELITe MGB Kit.
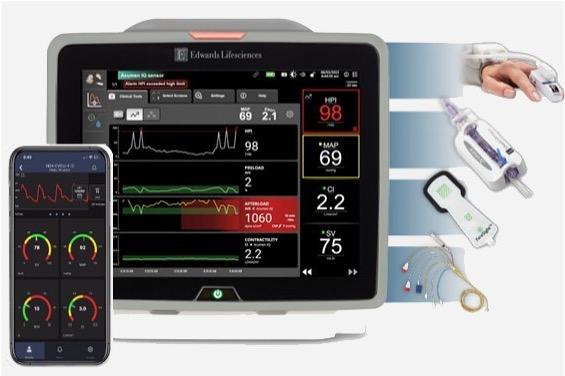
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, and Edwards Lifesciences (NYSE: EW), today announced a definitive agreement under which BD will acquire Edwards' Critical Care product group ("Critical Care"), a global leader in advanced monitoring solutions, for $4.2 billion in cash, unlocking new value creation opportunities and enhancing BD's portfolio of smart connected care solutions.

Maccura obtained the registration certificate of this product and was granted the invention patent in 2018. It is the first creatinine assay kit for anti-drug interference.

Watmind USA has been granted Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA) for its SpeedySwab Covid + FLU A&B Self-Test.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
