2.1.2 Registration of Instruments in Recent 2 Years
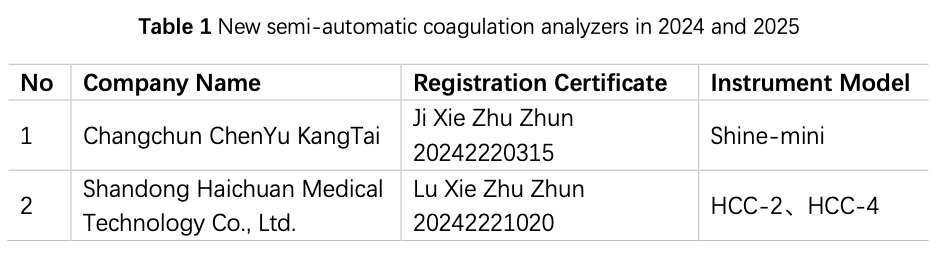
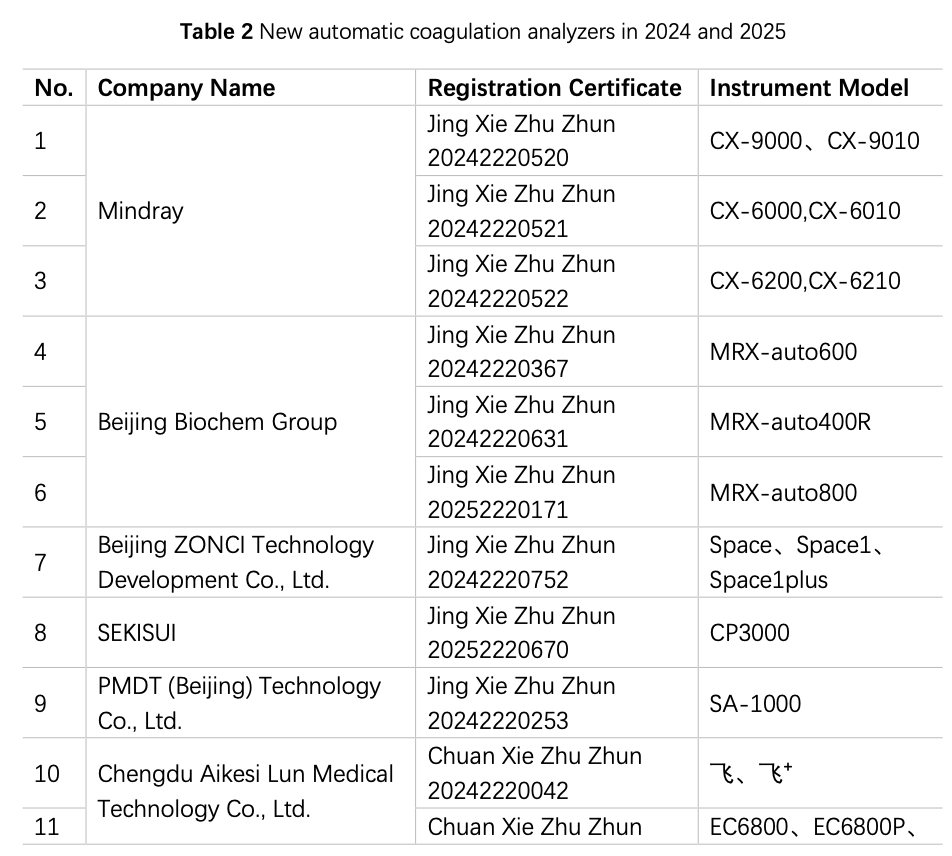
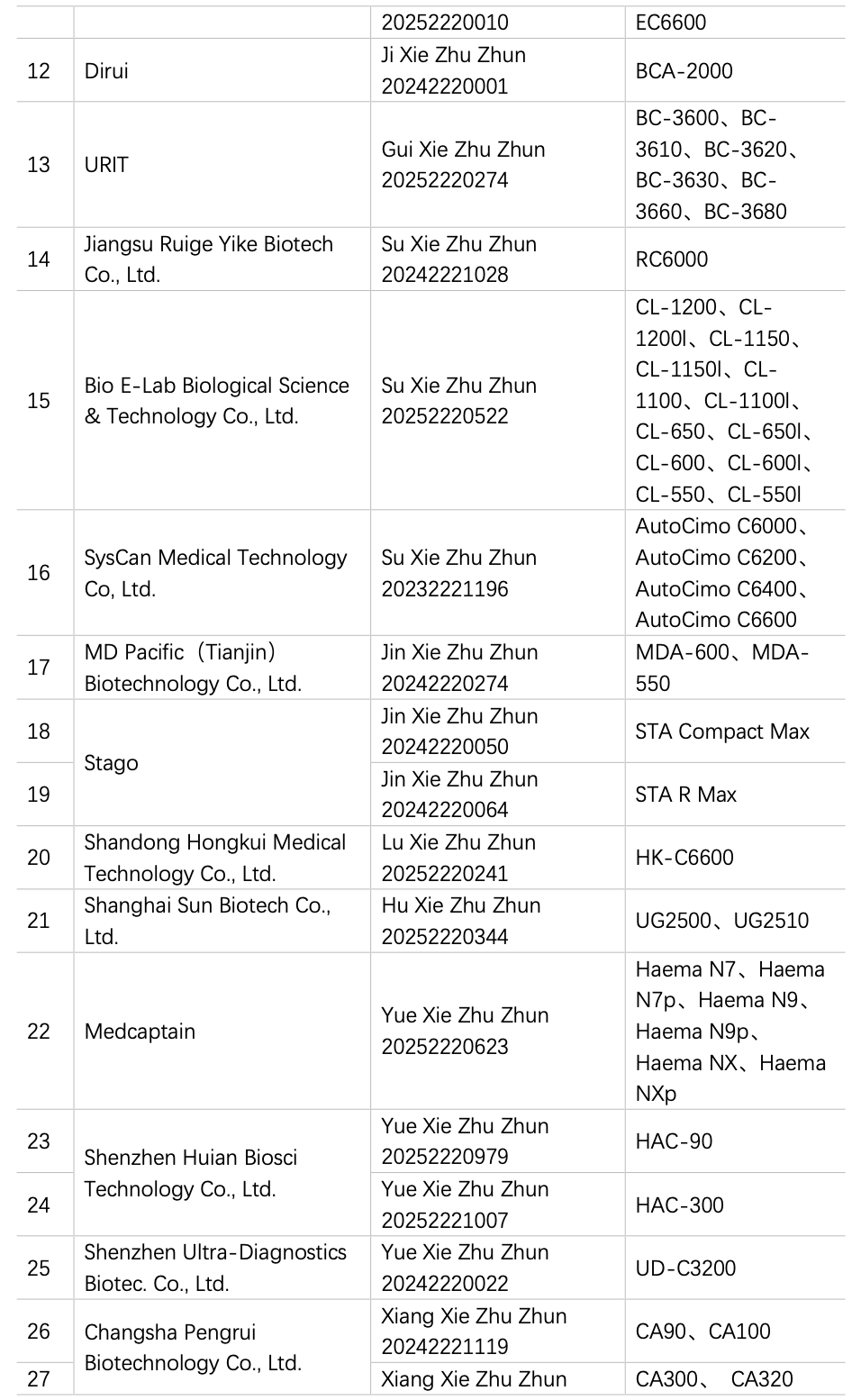
China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the National Medical Products Administration (hereinafter referred to as the NMPA), in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates for semi-automatic coagulation analyzers (Table 1), and 28 instruments by 20 manufacturers had obtained registration certificates for fully automated coagulation analyzers (Table 2), basically covering coagulation, chromogenic substrate, and immunoturbidimetric methods. The R&D and production of fully automated coagulation devices signify that China has made significant progress in automation and is actively catching up with the pace of mainstream manufacturers.

Last: In Vitro Diagnostic Industry in China - Diagnosis of Blood and Body Fluids IV
Next: In Vitro Diagnostic Industry in China - Diagnosis of Blood and Body Fluids VI

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
