<< Press release

Visitor registration for the 23rd China International In Vitro Diagnostic Expo by CACLP is now officially open.

As part of promotional activities leading up to CACLP 2026, a series of activities took place successfully in Xiamen last week. Among them, the Xiamen Forum on Empowering the In Vitro Diagnostics Industry and Exploring New Models of Collaboration was held on 21 November 2025.

A delegation from the China Association of In Vitro Diagnostics (CAIVD), the China Professional Community of Experimental Medicine (CPCEM), and CACLP visited Incheon for a four-day industry and academic exchange program.

Following its announcement to return to Xiamen in 2026, CACLP has received overwhelming enthusiasm from exhibitors worldwide. As of October 2025, more than 1,000 companies have confirmed their participation for next year’s event, reaffirming CACLP’s role as the world‘s largest IVD event and an important annual gathering of the global diagnostic community.
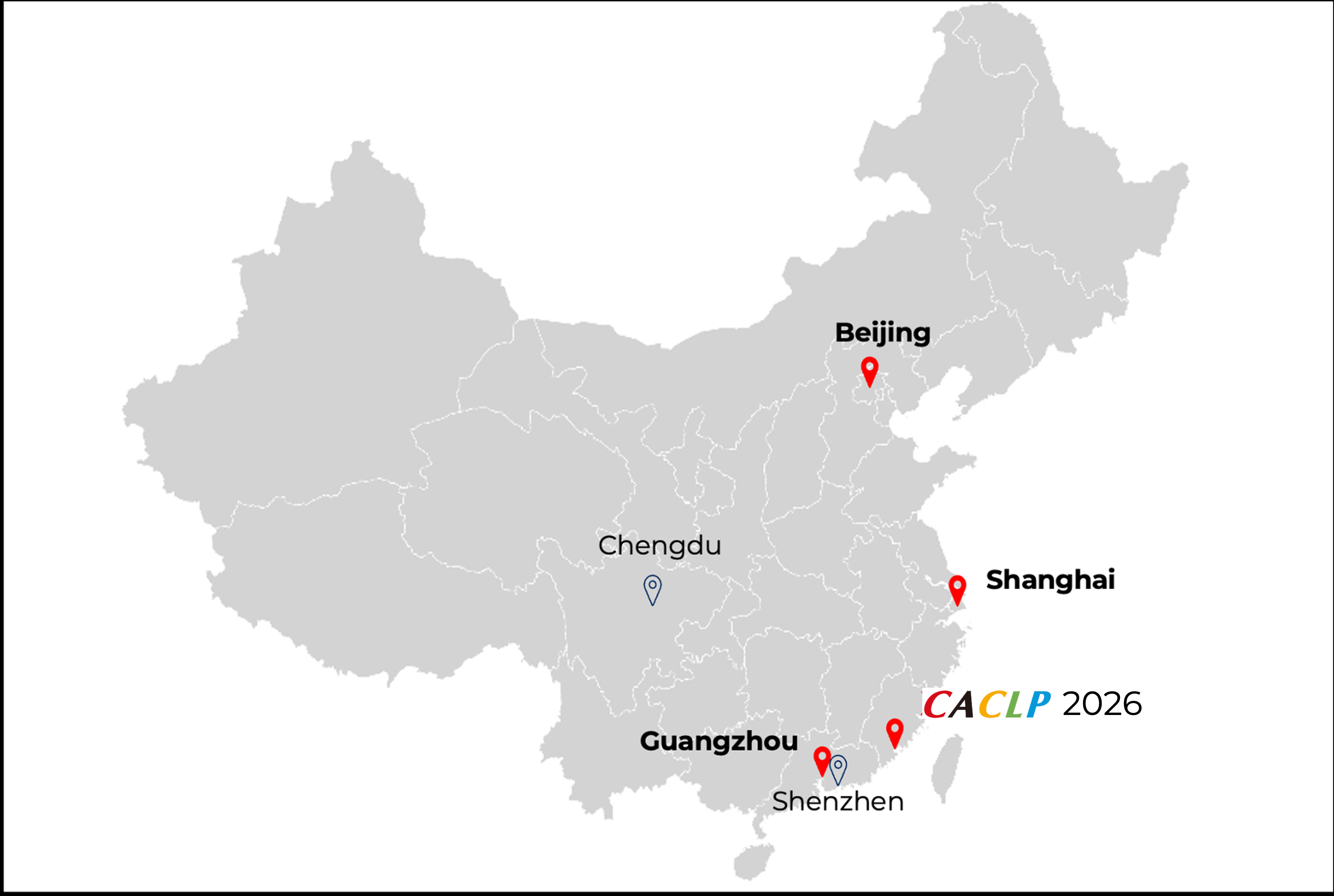
The 23rd China International In Vitro Diagnostic Expo by CACLP and its concurrent activities will return from 21–23 March 2026, at the Xiamen International Expo Center in Fujian Province, China.

On June 4 2025, Elsevier officially released the 2024 CiteScore, a key metric used to assess the impact of academic journals. VIEW, jointly sponsored by China Professional Community of Experimental Medicine (CPCEM) and published by John Wiley & Sons, achieved a CiteScore of 14.5 for 2024.

From 18-22 May 2025, Brussels, Belgium, hosted the 26th IFCC-EFLM EUROMEDLAB Congress of Clinical Chemistry and Laboratory Medicine and the 49th Annual Meeting of the Royal Belgian Society of Laboratory Medicine.

The 22nd China International In Vitro Diagnostics Expo by CACLP and the 5th China IVD Supply Chain Expo (CISCE) took place from 22–24 March 2025 in Hangzhou, achieving unprecedented scale and impact.
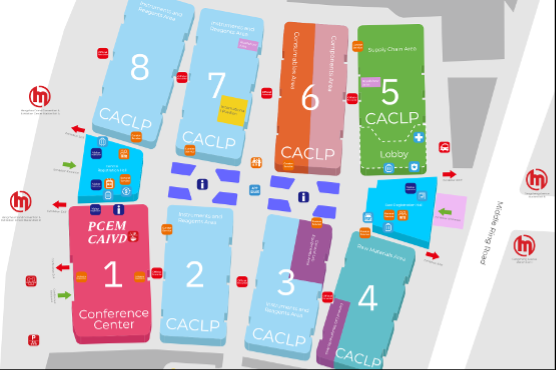
With just two weeks to go, anticipation is building for the 22nd China International In Vitro Diagnostic Expo by CACLP, the largest and most influential gathering of the IVD industry in China! Taking place 22-24 March 2025, at the Hangzhou Grand Convention & Exhibition Center, CACLP 2025 promises to be a world-class showcase of the latest innovations, professional networking and business opportunities.

The 7th China IVD Distribution Enterprise Forum is set to take place on 23 March 2025, at the Hangzhou Grand Convention and Exhibition Center.

<< Industry news

Sinopia Biosciences, Inc., a biotechnology company advancing novel therapeutics identified using its proprietary LEADS® drug discovery platform, announced today it has been awarded a research grant from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health (NIH), to further advance its data-driven drug discovery capabilities.

Biobeat Technologies, Ltd., developer of the first FDA-cleared, 24-hour ambulatory blood pressure monitoring (ABPM) system that is a patch-worn, cuff-less solution for diagnosis and treatment of hypertension, announced today the closing of a $50 million Series B equity financing.

Italian in vitro diagnostics firm Diasorin said on Sunday that the US Food and Drug Administration has issued 510(k) clearance and CLIA-waived the firm's first assay for use on the Liaison Nes platform.

Against the background of an increasingly aging global population, the incidence of thrombotic and hemostatic diseases, including cardiovascular diseases, remains high.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

The EU's Unified Patent Court last week ruled in favor of Myriad Genetics in its patent dispute with South Korean firm GXD-Bio.

Applied BioCode said on Monday that is has submitted a new nucleic acid extraction claim for its BioCode Respiratory Pathogen Panel (RPP) to the US Food and Drug Administration, with the goal of expanding use of the panel by clinical laboratories.
Following decades of cumulative efforts, a number of infectious disease tests and technologies broke new ground in 2025, hinting at rapid advances in the development of new systems and assays. But the past year also saw its fair share of upheaval, possibly suggesting more may be in store in 2026.

Chinese molecular diagnostics and precision medicine firm GenePlus Technology said on Sunday that it has filed for an initial public offering on the Hong Kong Stock Exchange.

Singlera Genomics said on Tuesday that it has entered into a research and distribution agreement with EU-based international medical company Pure Medical for its cell-free DNA detection products.

<< Video showcase


CACLP 2025 onsite - The Convergence of Laboratory Medicine and In Vitro Diagnostics


The 12th China IVD Industry Development Conference (CIIDC)


The 10th China Experimental Medicine Conference (CEMC)


International Laboratory Medicine and In Vitro Diagnostic Forum


The 11th China IVD Industry Development Conference (CIIDC)


The 9th China Experimental Medicine Conference (CEMC)


CACLP 2024 onsite - The Convergence of Laboratory Medicine and In Vitro Diagnostics

CACLP APP is available for download now!


Gentian at CACLP 2023 Onsite


in.vent Diagnostics at CACLP 2023 Onsite


Charming Group at CACLP 2023 Onsite


Dialab at CACLP 2023 Onsite


JTC at CACLP 2023 Onsite


Certest at CACLP 2023 Onsite


Yashraj Biotechnology at CACLP 2023 Onsite


Magtivio at CACLP 2023 Onsite


The 8th China Experimental Medicine Conference (CEMC)


The 10th China IVD Industry Development Conference (CIIDC)


CACLP 2023 onsite - The Convergence of Laboratory Medicine and In Vitro Diagnostics


CACLP's new website is launching!

<< Download centre

CACLP 2026 Exhibitor Manual



CACLP 2025 Post-show Report



CACLP 2025 Post-show Press Release



CACLP 2025 Exhibitor Manual



CACLP 2025 Floor Plan



CACLP 2025 Full Schedule



CACLP & CISCE 2024 Draw Global Attention to Innovations and Collaborations in IVD Industry



CACLP & CISCE to present the latest in IVD with 1300+ Exhibitors and 100+ Concurrent Activities this March in Chongqing.pdf



CACLP & CISCE 2024 - Floor Plan



CACLP & CISCE 2024 - Exhibitor Manual



Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
