
Those working in the industry will be aware that the implementation of the IVDR has been far from straightforward, and that there is still a lot of work to be done.

The European Union is proposing to delay the go-live date for the incoming medical device regulation pertaining to In Vitro Diagnostics for products that still need assessment of a notified body.
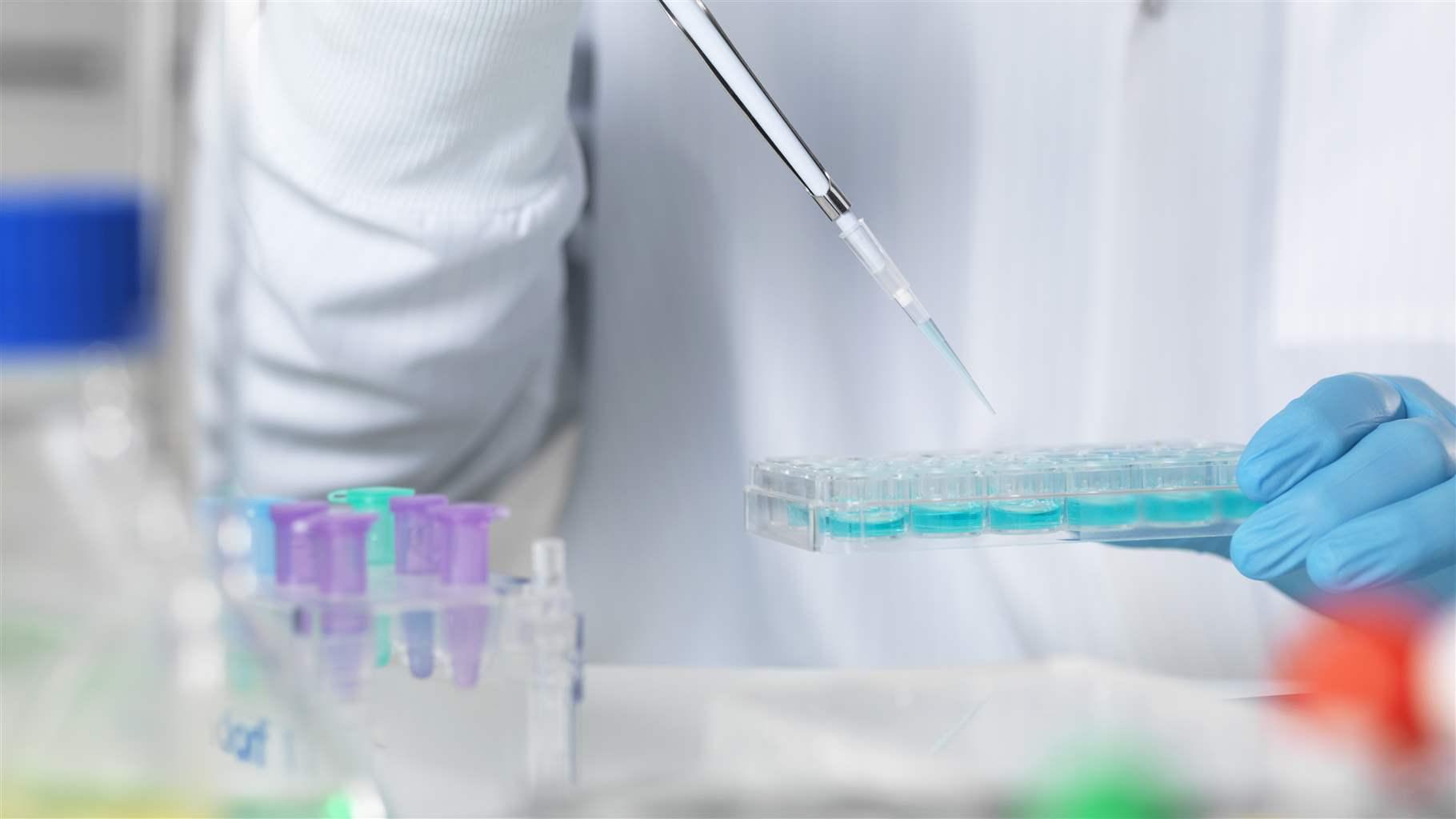
The Medical Device Coordination Group (MDCG) has published guidance MDCG 2021-21 “Guidance on performance evaluation of SARS-CoV-2 in vitro diagnostic medical devices” – the document is critical for all manufacturers who manufacture and have CE marked COVID-19 tests under the IVD Directive (IVDD).
✔ All (3)
✔ Press release (0)
✔ Industry news (3)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
